Simultaneous Saccharification and Fermentation (SSF) of Lignocellulosic Biomass
Lignocellulose Hydrolysis
Lignocellulosic biomass, primarily comprised of cellulose, hemicellulose, and lignin, is an abundant and renewable resource that holds great promise as a source of biofuels and renewable biobased chemicals and biomaterials. Lignocellulosic biomass can be processed in a number of ways, one is through the hydrolysis of the structural polysaccharides (cellulose and hemicellulose) into their constituent sugars, a reaction commonly facilitated by acid or enzymes, followed by the fermentation of these sugars by yeast or other microorganisms.Click below to read more about the hydrolysis of lignocellulosic biomass.
Get more info...Biomass Hydrolysis
Enzymatic Hydrolysis
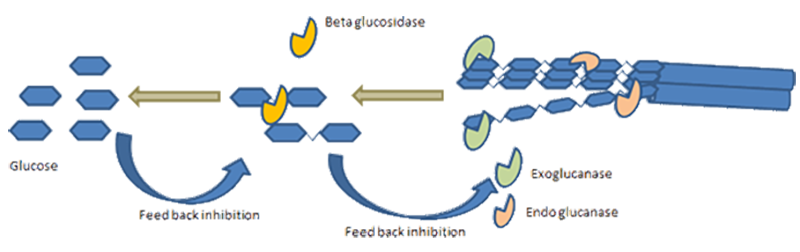
In enzymatic hydrolysis cellulases and hemicellulases play a critical role, working synergistically to cleave the glycosidic linkages in cellulose and hemicellulose, respectively. However, depending on the type of pretreatment process involved, hydrolysis of hemicellulose may not be necessary, since it may have already taken place in the pretreatment leading to the hemicellulose sugars being in the liquid output of the pretreatment with the solid residue mostly containing cellulose (plus lignin, again dependent on the type of pretreatment).The enzymatic hydrolysis step can be carried out under relatively mild conditions, which makes it a more energy-efficient and environmentally friendly approach compared to chemical hydrolysis methods.
SSF Process and its Advantages
Simultaneous Saccharification and Fermentation (SSF) is a process for the production of sugar-derived products (such as bioethanol) from lignocellulosic biomass. The process involves the concurrent breakdown (hydrolysis) of cellulose (and hemicellulose, if present) into monomeric sugars (saccharification), and the conversion of these sugars into products via fermentation. This process is distinct from the Separate Hydrolysis and Fermentation (SHF) method, in which saccharification and fermentation occur in separate stages.Some of the advantages of the Simultaneous Saccharification and Fermentation (SSF) approach are described below:
- Reduction of End-Product Inhibition - In SSF, as sugars are released from the cellulose and hemicellulose, they are immediately consumed by the microorganisms and converted into the target product (e.g. bioethanol). This rapid conversion helps to mitigate the potential inhibition of cellulolytic enzymes that can occur when sugar concentrations become too high.
- Process Efficiency and Cost-Effectiveness - By combining the hydrolysis and fermentation stages, SSF reduces the total process time, potentially leading to lower capital and operational costs. Furthermore, it eliminates the need for separate reactors for hydrolysis and fermentation, reducing equipment costs.
- Reduced Risk of Contamination - The shorter process time and fewer stages in SSF decrease the opportunities for contamination compared to processes with separate stages (e.g. Separate Hydrolysis and Fermentation).
Disadvantages of SSF
- Difficulty in Optimising Conditions - A significant challenge in SSF is the difficulty in optimising conditions for both hydrolysis and fermentation. The optimal temperatures for enzymatic hydrolysis and yeast fermentation do not coincide, which may result in suboptimal performance of one or both processes.
- Potential for Enzyme Inactivation - Fermentation often takes place at lower temperatures that are not optimal for the enzymes, which could decrease the hydrolysis rate.
- Limited choice of Fermenting Microorganisms - Not all microorganisms can withstand the conditions required for enzymatic hydrolysis, which can limit the choice of microorganisms for fermentation.
Fed-Batch SSF
Fed-batch Simultaneous Saccharification and Fermentation (SSF) is a modification of the traditional SSF process which can, in certain circumstances, improve process efficiencies. In the traditional SSF process, all the components – the lignocellulosic biomass, enzymes, and yeast – are mixed together at the beginning of the process. However, in fed-batch SSF these components are not all added at once. Instead, the lignocellulosic biomass is added in incremental portions or "batches" to the fermenter over the course of the fermentation. By gradually feeding the biomass into the fermenter, it is possible to maintain a more consistent level of sugars throughout the process, which can help to optimize yeast activity and product yields. In addition, this approach can help to alleviate issues related to high solid loading, such as mixing problems and high viscosity.Separate Hydrolysis & Fermentation (SHF)
Separate Hydrolysis and Fermentation (SHF) involves separate stages of enzymatic hydrolysis and fermentation, occuring in separate reactors.Advantages of SSF over SHF:
- Reduced Inhibition - In SSF, as soon as the cellulose and hemicellulose are broken down into monomers, they are immediately consumed by the fermenting organisms. This helps to prevent the build-up of sugars that can inhibit the enzymes carrying out the hydrolysis.
- Allows for Fed-Batch Processing - Related to the previous point, the ongoing removal of sugars in SSF means that fed-batch processing is possible. In contast, the build-up of monomers, and subsequent product inhibition on enzyme activities, means that the fed-batch approach is not suitable for SHF processes.
- Time and Cost Savings - SSF can be more cost-effective and quicker than SHF because it requires fewer process steps and reduces the need for separate reactors.
- Reduced Risk of Contamination - The combined process and shorter overall duration decrease the chances for contamination.
- Suboptimal Conditions - The optimal temperatures for hydrolysis and fermentation differ. Since both processes happen simultaneously in SSF, it is hard to find an optimal temperature that maximizes both processes, leading to potential inefficiencies. In contrast, in SHF the separate nature of the hydrolysis and fermentation stages allows each process to be optimized independently for parameters such as temperature, pH, and enzyme concentration.
- Limited Microorganisms - Not all fermenting organisms can tolerate the conditions required for effective enzymatic hydrolysis, limiting the choice of organisms that can be used in the SSF process.
- Challenging Process Control - It is more difficult to control the individual stages of the process in SSF as they are happening at the same time.
Get more info...SHF
Consolidated Bioprocessing (CBP)
Like SSF, in Consolidated Bioprocessing (CBP) the hydrolysis, and fermentation steps occur in the same reactor. However, unlike SSF, in CBP the production of enzymes also takes place in the same reactor. CBP has the potential to significantly reduce costs and simplify the biofuel production process. However, the implementation of CBP is currently challenging because it requires a single microorganism, or a consortium of microorganisms, that can efficiently perform all the required functions.Advantages of SSF over CBP:
- Established Processes and Microorganisms - SSF uses well-established processes and microorganisms for the saccharification and fermentation stages. This familiarity can lead to more predictable outcomes and potentially easier process optimisation.
- Intermediate Control - SSF allows for control over the intermediate steps, offering potential adjustments based on real-time process dynamics.
- Flexibility in Microorganism Selection - SSF provides flexibility in choosing different microorganisms for saccharification and fermentation, potentially leading to higher efficiency.
- More-Established Technology - The development of CBP technologies is still at the R&D stage whilst SSF technologies have been deployed at the commercial scale.
- Suboptimal Process Conditions - The conditions that are ideal for enzymatic hydrolysis are not typically ideal for microbial fermentation, which may lead to suboptimal efficiency in one or both processes in SSF.
- Process Complexity - SSF, while simpler than Separate Hydrolysis and Fermentation (SHF), is still more complex than CBP due to the need for separate stages.
- Potential for Enzyme Inactivation - The temperature and pH conditions optimal for fermentation may not be ideal for the enzymes used in saccharification, potentially leading to reduced enzymatic activity.
- Higher CAPEX and OPEX - Unlike in CBP, SSF processes require that enzymes are added at the hydrolysis stage. Either these SSF enzymes have to be purchased, adding significantly to OPEX, or they need to be manufactured on-site, adding to OPEX and CAPEX.
Get more info...CBP
1. Understanding Your Requirements
Prior to undertaking bioprocess projects we learn from our clients what their targets are from the process as well as whether there are any restrictions or requirements that may need to form the boundaries of the work that we undertake. These help to guide us to then prepare a potential bioprocess development project.
2. Detailed Feedstock Analysis
In cases where you have already selected a feedstock for the bioprocess, we would then undertake a detailed compositional analysis (P10 or, ideally, P19) of representative samples of that feedstock.
In cases where the feedstock has not yet been selected we can review your list of candidate feedstocks, selecting top candidates based on our prior experience in their analysis and bioprocessing. If you do not have a list of candidate feedstocks then we can provide one, based on your location and the requirements outlined in Stage 1. We would then analyse in detail these priority feedstocks and come to a decision, based on the compositional data and other relevant factors (e.g. price, supply, consistency etc.) on a selected feedstock for the project.
At this point of the project, the Celignis Bioprocess team typically meet to discuss and prepare a project proposal for the development of a SSF bioprocess from this feedstock. After this proposal is reviewed by the client, and revised if needed, we are then ready to start work on the next stages.
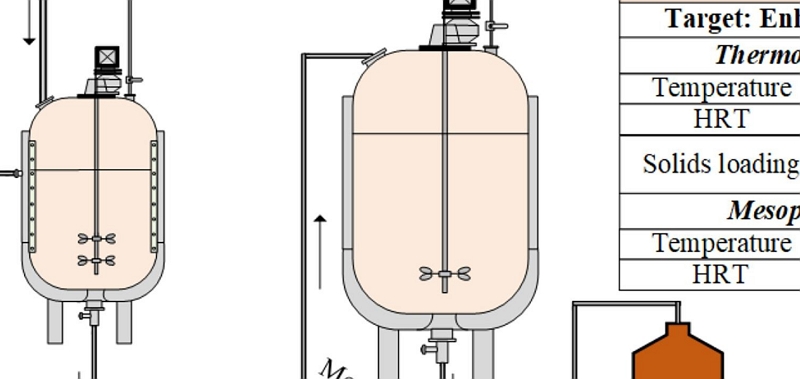
3. Pretreatment (Lab-Scale)
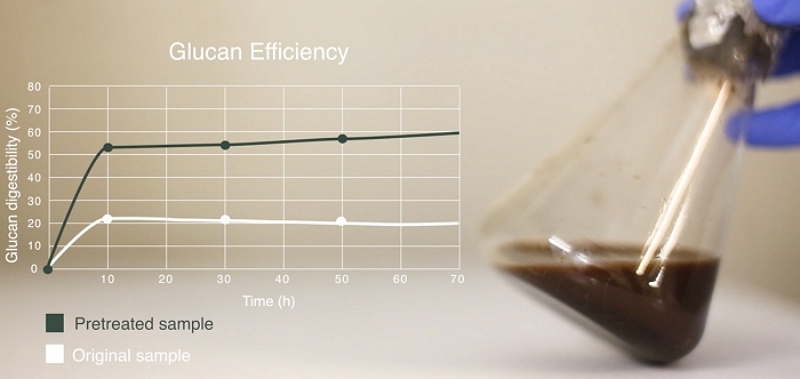
If our project involves the SSF-processing of a virgin biomass feedstock then it is likely that a pretreatment stage will be necessary in order to provide a suitable substrate for subsequent hydrolysis and fermentation. Hence, this stage of the project will involve undertaking a number of pretreatment experiments, covering a variety of process conditions. We follow a scientifically-based Design of Experiments (DoE) protocol where the criteria and boundaries for this DoE are formulated in close collaboration with our clients, considering the chemistry of the feedstock(s) and our understandings of the mechanisms of biomass pretreatment.
We usually recommend that these initial optimisation experiments are undertaken at the lab-scale (around TRL3) in order to reduce costs and the length of the project. For each experiment we analyse the solid and liquid outputs of the pretreatment process, leading to a detailed data-set where effects of process conditions on the yield and composition of the various streams can be explored and mapped.
We can also undertake a second iteration of lab-scale experiments in order to fine-tune the conditions based on the knowledge gained in the initial experiments.
4. SSF Optimisation
At this stage the focus is on optimising the SSF of the pretreated biomass. This will involve evaluating the effects of varying a number of different process conditions (e.g. solid-loading, enzyme-loading, enzyme-type, yeast-type and loading, temperature, reaction times, mixing). There may also need to be experiments comparing Fed-Batch SSF with standard SSF.
Hence, this stage of the project involves a DoE being undertaken (like in Stage 3) where these conditions are narrowed down. Again, these optimisation experiments are usually undertaken at the lab-scale in order to accelerate the outputs and reduce project costs.
This stage usually follows the pretreatment optimisation activities, however it is possible that there can be some overlap in order to reach the final project outputs more quickly.
5. Product Recovery
Based on the outputs of the prior lab-scale Stages we can optimise the methods employed for separating and purifying the target product from the fermentation Stage. We can also potentially look at the recovery of other compounds from the broth.
It is possible for this Stage to run alongside Stage 4.
6. Valorisation of Remaining Biomass
Depending on the pretreatment and SSF processess employed in the project, we can look at valorising those biomass components that are not hydrolysed to sugars and fermented to your target product.
For example, we can analyse the enzymatic hydrolysis residue for its suitability for combustion. In another scenario we can evaluate the potential for valorising the hemicellulose sugars that are hydrolysed in the dilute-acid-pretreatment stage.
7. Validation at Higher TRLs
Once we have concluded our optimisation of the SSF process conditions at the lab-scale we can then test those conditions at higher technology readiness levels (TRLs). The scales at which we can operate are dependent on the type of technology employed, but can reach up to 100 litres.
We have all of the necessary downstream equipment to efficiently handle the solid and liquid streams arising from these scaled-up activities.
If we find that there are differences between the yield and compositions of the different streams, compared with our lab-scale experiments, then we can explore the potential reasons for these and work on final tweaks to optimise the bioprocess for higher TRLs.
8. Technoeconomic Analysis (TEA)
The Celignis team, including Oscar our chief TEA expert, can undertake a detailed technoeconomic analysis of the developed process. We apply accurate and realistic costing models to determine the CAPEX and OPEX of simulated and pilot scale processes which are then used to determine key economic indicators such as IRR, NPV and payback periods.
Within these TEAs we can undertake sensitivity analyses to assess the effect of variable costs and revenues on the commercial viability of the process.
Our preferred approach is to include TEA studies at each stage of the development of the bioprocess, so that the process can be optimised in a commercially-relevant way, followed by a more detailed TEA after the process has been optimised and tested at higher TRL levels.
Click here to read more about the technoeconomic analysis (TEA) services offered by Celignis.

Lalitha Gottumukkala
Founder of Celignis Bioprocess, CIO of Celignis
PhD
<p style="text-align: left;">Has a deep understanding of all biological and chemical aspects of bioproceses. Has developed Celignis into a renowned provider of bioprocess development services to a global network of clients.</p>

Oscar Bedzo
Bioprocess Project Manager & Technoeconomic Analysis Lead
PhD
<p style="text-align: left;">A dynamic, purpose-driven chemical engineer with expertise in bioprocess development, process design, simulation and techno-economic analysis over several years in the bioeconomy sector.</p>

Dan Hayes
Celignis CEO And Founder
PhD (Analytical Chemistry)
<p style="text-align: left;">Dreamer and achiever. Took Celignis from a concept in a research project to being the bioeconomy's premier provider of analytical and bioprocessing expertise.</p>