Fermentation for Biobased Chemicals and Products
Here at Celignis our team is highly experienced in numerous types of fermentations and can help you determine and optimise the potenital yields of an array of different fermentation products. If you already have a technology, e.g. pre-treatment and/or hydrolysis, that produces a sugars-containing liquid then we can undertake fermentation tests directly on that. Alternatively, if you are starting with a feedstock and looking for the best approach to get your targetted fermentation product(s) in high yield then we can help you optimise the whole process, covering pre-treatment, hydrolysis and the subsequent fermentation.
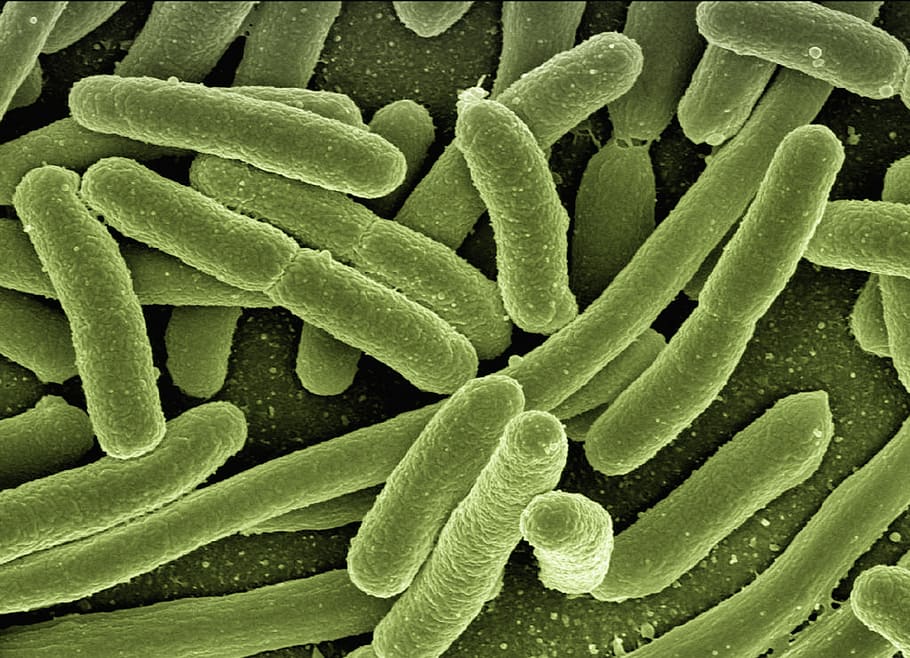
Bacterial Fermenation
Yeast and Fungal Fermenation
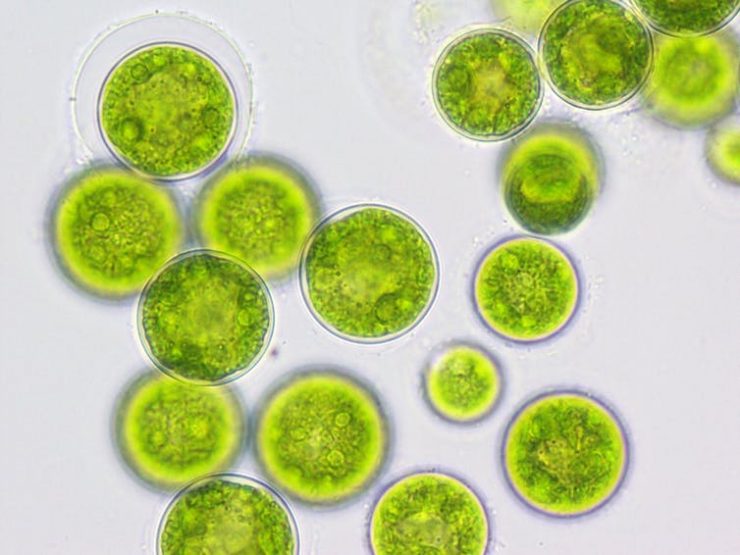
Algal Fermenation
1. Bacterial Fermentation
Get more info...Bacterial Fermentation
Lactic Acid Fermentation - At Celignis we have expertise and experience in screening lactic acid bacteria for the selection of substrate- and product-tolerant strains. We can also develop: fed-batch strategies to achieve high cell mass, and in situ product recovery techniques to separate lactic acid from the fermentation broth. We will work with you and develop bespoke lactic acid fermentation methods for your feedstock or industrial waste streams.
2. Yeast and Fungal Fermentation
Get more info...Yeast Fermentation
Ethanol Production - We can use a variety of yeast types to produce bioethanol. We can ferment the liquids obtained in a prior hydrolysis step (separate hydrolysis and fermentation, SHF) or we can combine the hydrolysis and fermentation stages using simultaneous saccharification and fermentation (SSF) or simultaneous saccharification and co-fermentation (SSCF) processes.
3. Microalgal Fermentation
Get more info...Microalgae Fermentation
With regards to the development of fermentation bioprocesses, the Celignis Bioprocess team members with the most experience in undertaking such projects are listed below. Feel free to contact them directly to discuss potential projects.

Lalitha Gottumukkala
Founder of Celignis Bioprocess, CIO of Celignis
PhD
<p style="text-align: left;">Has a deep understanding of all biological and chemical aspects of bioproceses. Has developed Celignis into a renowned provider of bioprocess development services to a global network of clients.</p>

Oscar Bedzo
Bioprocess Project Manager & Technoeconomic Analysis Lead
PhD
<p style="text-align: left;">A dynamic, purpose-driven chemical engineer with expertise in bioprocess development, process design, simulation and techno-economic analysis over several years in the bioeconomy sector.</p>

Dan Hayes
Celignis CEO And Founder
PhD (Analytical Chemistry)
<p style="text-align: left;">Dreamer and achiever. Took Celignis from a concept in a research project to being the bioeconomy's premier provider of analytical and bioprocessing expertise.</p>
Six conceptual process scenarios for the production of biobutanol from lignocellulosic biomass through acetone?butanol?ethanol (ABE) fermentation, using reported data on process performances, were developed with ASPEN Plus® V8.2 software. The six scenarios covered three fermentation strategies, i.e. batch separate hydrolysis and fermentation (SHF), continuous SHF, and batch simultaneous saccharification and fermentation (SSF) integrated with gas stripping (GS). The two downstream processing options considered were double?effect distillation (DD) and liquid?liquid extraction and distillation (LLE&D). It was found that the SSF?GS/DD scenario was the most energy efficient with a liquid fuel efficiency of 24% and an overall efficiency of 31%. This was also the scenario with the best economic outcome, with an internal rate of return (IRR) of 15% and net present value (NPV) of US$387 million. The SSF?GS/DD scenario was compared to a similar molasses process, based on the product flow rates, and it was found that the molasses process was more energy efficient with a gross energy value (GEV) of 23?MJ?kg1 butanol compared to ?117?MJ?kg1 butanol for the lignocellulosic process. In addition, the molasses?based process was more profitable with an IRR of 36% compared to 21%. However, the energy requirements for the molasses process were supplied from fossil fuels, whereas for the lignocellulose processes a portion of the feedstock was diverted to provide process energy. Improved environmental performance is therefore associated with the lignocellulosic process. | |
Disposal of waste sludges produced in large amounts in the pulp and paper industry imposes significant environmental and economical problems. One strategy to address these issues involves revalorization of paper mill sludges by their application as substrates for microbial production of biotechnologically relevant enzymes. The application of lignocellulolytic enzymes in paper, textile and bioenergy industries is encouraged in order to decrease chemicals and energy consumptions. In the following work, deinking sludge was assessed as a substrate for production of lignocellulases. Based on the results of growth and activity screenings, Pleurotus ostreatus PLAB was chosen as the most promising candidate among 30 tested strains and its secretome was further studied by quantitative enzyme assays and mass spectrometry. While endoglucanase and xylanase activities detected in P. ostreatus secretome produced on deinking sludge were similar to activities of cultures grown on other lignocellulosic substrates, average laccase activity was significantly higher (46?000 U/kg DIS). Mass spectrometry identification of the most prominent proteins in the secretome of the target strain confirmed that significant amounts of different lignin-modifying oxidases were produced on this substrate despite its low lignin content, indicating the presence of other inducible compounds. The findings of this study suggest deinking sludge may represent a good substrate for fungal production of the aforementioned enzymes with broad biotechnological applications, including bioremediation, paper and bioenergy industries. | |
Biobutanol has gained attention as an alternative renewable transportation fuel for its superior fuel properties and widespread applications in chemical industry, primarily as a solvent. Conventional butanol fermentation has drawbacks that include strain degeneration, end-product toxicity, by-product formation, low butanol concentrations and high substrate cost. The complexity of Clostridium physiology and close control between sporulation phase and ABE fermentation has made it demanding to develop industrially potent strains. In addition to the isolation and engineering of superior butanol producing bacteria, the development of advanced cost-effective technologies for butanol production from feedstock like lignocellulosic biomass has become the primary research focus. High process costs associated with complex feedstocks, product toxicity and low product concentrations are few of the several bioprocess challenges involved in biobutanol production. The article aims to assess the challenges in lignocellulosic biomass to biobutanol conversion and identify key process improvements that can make biobutanol commercially viable. | |
Paper sludge (PS) from the paper and pulp industry consists primarily of cellulose and ash and has significant potential for ethanol production. Thirty-seven PS samples from 11 South African paper and pulp mills exhibited large variation in chemical composition and resulting ethanol production. Simultaneous saccharification and fermentation (SSF) of PS in fed-batch culture was investigated at high solid loadings and low enzyme dosages. Water holding capacity and viscosity of the PS influenced ethanol production at elevated solid loadings of PS. High viscosity of PS from virgin pulp mills restricted the solid loading to 18% (w/w) at an enzyme dosage of 20 FPU/gram dry PS (gdPS), whereas an optimal solid loading of 27% (w/w) was achieved with corrugated recycle mill PS at 11 FPU/gdPS. Ethanol concentration and yield of virgin pulp and corrugated recycle PS were 34.2 g/L at 66.9% and 45.5 g/L at 78.2%, respectively. | |
Paper sludge samples collected from recycling mills exhibited high ash content in the range of 54.59%–65.50% and glucose concentrations between 21.97% and 31.11%. Washing the sludge reduced the total ash content to between 10.7% and 19.31% and increased the concentration of glucose, xylose and lignin. Samples were screened for ethanol production and fed-batch simultaneous saccharification and fermentation (SSF) was optimised for the washed samples that resulted in highest and lowest ethanol concentrations. Maximum ethanol concentrations of 57.31 g/L and 47.72 g/L (94.07% and 85.34% of the maximum theoretical yield, respectively) was predicted for high and low fermentative potential samples, respectively, and was experimentally achieved with 1% deviation. A generic set of process conditions were established for the conversion of high ash-containing paper sludge to ethanol. Techno-economic analysis based on three different revenue scenarios, together with Monte Carlo analysis revealed 95% probability of achieving IRR values in excess of 25% at a paper sludge feed rate of 15 t/d. Feed rates of 30 t/d and 50 t/d exhibited a cumulative probability of 100%. This study presents the technical feasibility and economic viability of paper mills expansion towards bioethanol production from paper sludge. | |
Next-generation biofuels from renewable sources have gained interest among research investigators, industrialists, and governments due to major concerns on the volatility of oil prices, climate change, and depletion of oil reserves. Biobutanol has drawn signicant attention as an alternative transportation fuel due to its superior fuel properties over ethanol. e advantages of butanol are its high energy content, better blending with gasoline, less hydroscopic nature, lower volatility, direct use in convention engines, low corrosiveness, etc. Butanol production through (acetone, butanol, and ethanol) ABE fermentation is a well-established process, but it has several drawbacks like feedstock cost, strain degeneration, product toxicity, and low product concentrations. Lignocellulosic biomass is considered as the most abundant, renewable, low-cost feedstock for biofuels. Production of butanol from lignocellulosic biomass is more complicated due to the recalcitrance of feedstock and inhibitors generated during the pretreatment and hydrolysis process. Advanced fermentation and product recovery techniques are being researched to make biobutanol industrially viable. | |
The aim of this work was to evaluate the biosurfactants produced by the yeast Pseudozyma sp. NII 08165 for enhancing the degradation of crude oil by a model hydrocarbon degrading strain, Pseudomonas putida MTCC 1194. Pseudozyma biosurfactants were supplemented at various concentrations to the P. putida culture medium containing crude oil as sole carbon source. Supplementation of the biosurfactants enhanced the degradation of crude oil by P. putida; the maximum degradation of hydrocarbons was observed with a 2.5 mg L?1 supplementation of biosurfactants. Growth inhibition constant of the Pseudozyma biosurfactants was 11.07 mg L?1. It was interesting to note that Pseudozyma sp. NII 08165 alone could also degrade diesel and kerosene. Culture broth of Pseudozyma containing biosurfactants resulted up to ?46% improvement in degradation of C10–C24 alkanes by P. putida. The enhancement in degradation efficiency of the bacterium with the culture broth supplementation was even more pronounced than that with relatively purer biosurfactants. | |
Bioethanol and biobutanol hold great promise as alternative biofuels, especially for transport sector, because they can be produced from lignocellulosic agro-industrial residues. From techno-economic point of view, the bioprocess for biofuels production should involve minimal processing steps. Consolidated bioprocessing (CBP), which combines various processing steps such as pretreatment, hydrolysis and fermentation in a single bioreactor, could be of great relevance for the production of bioethanol and biobutanol or solvents (acetone, butanol, ethanol), employing clostridia. For CBP, Clostridium holds best promise because it possesses multi-enzyme system involving cellulosome and xylanosome, which comprise several enzymes such as cellulases and xylanases. The aim of this article was to review the recent developments on enzyme systems of clostridia, especially xylanase and cellulase with an effort to analyse the information available on molecular approaches for the improvement of strains with ultimate aim to improve the efficiencies of hydrolysis and fermentation. | |
Growth inhibition kinetics of a novel non-acetone forming butanol producer, Clostridium sporogenes BE01, was studied under varying concentrations of acetic and formic acids in rice straw hydrolysate medium. Both the organic acids were considered as inhibitors as they could inhibit the growth of the bacterium, and the inhibition constants were determined to be 1.6 and 0.76 g/L, respectively, for acetic acid and formic acid. Amberlite resins—XAD 4, XAD 7, XAD 16, and an anion exchange resin—Seralite 400 were tested for the efficient removal of these acidic inhibitors along with minimal adsorption of sugars and essential minerals present in the hydrolysate. Seralite 400 was an efficient adsorbent of acids, with minimal affinity towards minerals and sugars. Butanol production was evaluated to emphasize the effect of minerals loss and acids removal by the resins during detoxification. | |
Biobutanol from lignocellulosic biomass has gained much attention due to several advantages over bioethanol. Though microbial production of butanol through ABE fermentation is an established technology, the use of lignocellulosic biomass as feedstock presents several challenges. In the present study, biobutanol production from enzymatic hydrolysate of acid pretreated rice straw was evaluated using Clostridium sporogenes BE01. This strain gave a butanol yield of 3.43 g/l and a total solvent yield of 5.32 g/l in rice straw hydrolysate supplemented with calcium carbonate and yeast extract. Hydrolysate was analyzed for the level of inhibitors such as acetic acid, formic acid and furfurals which affect the growth of the organism and in turn ABE fermentation. Methods for preconditioning the hydrolysate to remove toxic end products were done so as to improve the fermentation efficiency. Conditions of ABE fermentation were fine tuned resulting in an enhanced biobutanol reaching 5.52 g/l. | |
Biobutanol from lignocellulosic biomass has gained much attention due to several advantages over bioethanol. Though microbial production of butanol through ABE fermentation is an established technology, the use of lignocellulosic biomass as feedstock presents several challenges. In the present study, biobutanol production from enzymatic hydrolysate of acid pretreated rice straw was evaluated using Clostridium sporogenes BE01. This strain gave a butanol yield of 3.43 g/l and a total solvent yield of 5.32 g/l in rice straw hydrolysate supplemented with calcium carbonate and yeast extract. Hydrolysate was analyzed for the level of inhibitors such as acetic acid, formic acid and furfurals which affect the growth of the organism and in turn ABE fermentation. Methods for preconditioning the hydrolysate to remove toxic end products were done so as to improve the fermentation efficiency. Conditions of ABE fermentation were fine tuned resulting in an enhanced biobutanol reaching 5.52 g/l. | |
The aim of this work was to study the production of exopolysaccharide (EPS) from a novel ustilaginomycetes yeast strain Pseudozyma sp. NII 08165. The culture produced 3.5g/l EPS on fourth day of fermentation in a glucose-based medium. The structural characterization revealed that the EPS was a polymer of glucose, galactose and mannose in the ratio of 2.4:5.0:2.6 with a molecular weight of 1.7MDa. The pseudoplastic behaviour of aqueous EPS with a thermal stability up to 220 C indicated its potential utility as a thickening or gelling agent in food industry. SEM studies of the EPS showed that it had compact film-like structure, which could make it a useful in preparing plasticized films. The AFM studies showed that EPS had spike-shaped microstructure. Physical properties of the exopolysaccharide determined further indicated its possible potential in different industrial applications. | |
Aspergillus niger NII-08121/MTCC 7956 exhibited differences in expression of ?-glucosidase (BGL) in response to carbon sources provided in the medium. Activity staining with methyl umbelliferyl ?-d-glucopyranoside (MUG) indicated that four different isoforms of BGL were expressed when A. niger was grown under submerged fermentation with either lactose or cellulose, whereas only two were expressed when wheat bran or rice straw was used as the carbon source. Among the four isoforms of BGL expressed during lactose supplementation, two were found to retain 92% and 82% activity respectively in presence of 250 mM glucose in the MUG assay. The major ?-glucosidase (BGL1) was purified to homogeneity by electro elution from a Native PAGE gel. The purified 120 kDa protein was active at 50 °C and was stable for 48 h without any loss of activity. The optimum pH and temperature were 4.8 and 70 °C respectively. | |
Rice straw is an attractive lignocellulosic material for bioethanol production since it is one of the most abundant renewable resources. It has several characteristics, such as high cellulose and hemicelluloses content that can be readily hydrolyzed into fermentable sugars. But there occur several challenges and limitations in the process of converting rice straw to ethanol. The presence of high ash and silica content in rice straw makes it an inferior feedstock for ethanol production. One of the major challenges in developing technology for bioethanol production from rice straw is selection of an appropriate pretreatment technique. The choice of pretreatment methods plays an important role to increase the efficiency of enzymatic saccharification thereby making the whole process economically viable. The present review discusses the available technologies for bioethanol production using rice straw. | |
Biomass feedstock having less competition with food crops are desirable for bio-ethanol production and such resources may not be localized geographically. A distributed production strategy is therefore more suitable for feedstock like water hyacinth with a decentralized availability. In this study, we have demonstrated the suitability of this feedstock for production of fermentable sugars using cellulases produced on site. Testing of acid and alkali pretreatment methods indicated that alkali pretreatment was more efficient in making the sample susceptible to enzyme hydrolysis. Cellulase and ?-glucosidase loading and the effect of surfactants were studied and optimized to improve saccharification. Redesigning of enzyme blends resulted in an improvement of saccharification from 57% to 71%. A crude trial on fermentation of the enzymatic hydrolysate using the common baker’s yeast Saccharomyces cerevisiae yielded an ethanol concentration of 4.4 g/L. | |
Global Recognition as Bioprocess Experts
Extraction
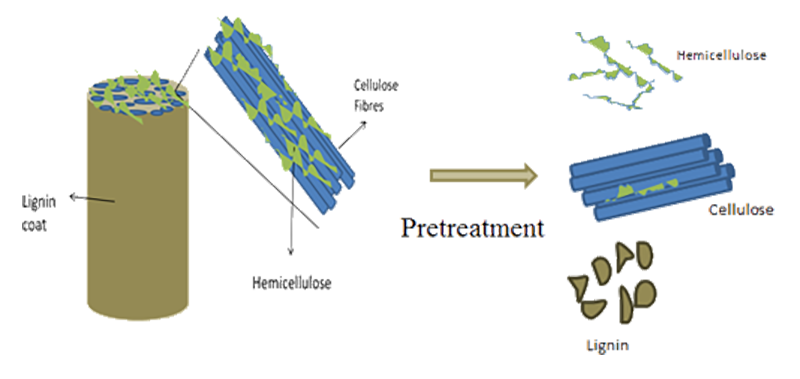
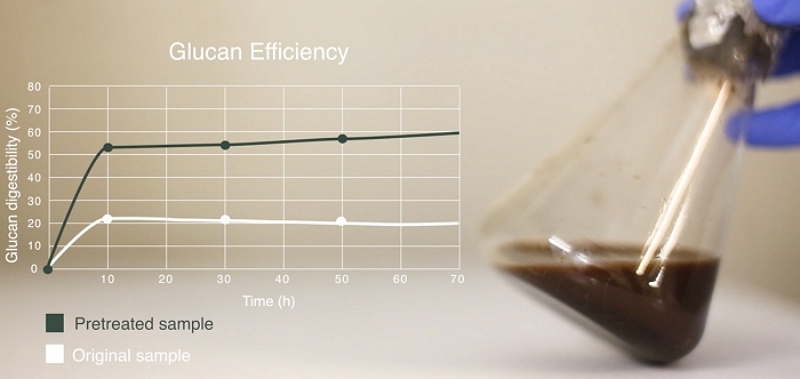
Pretreatment
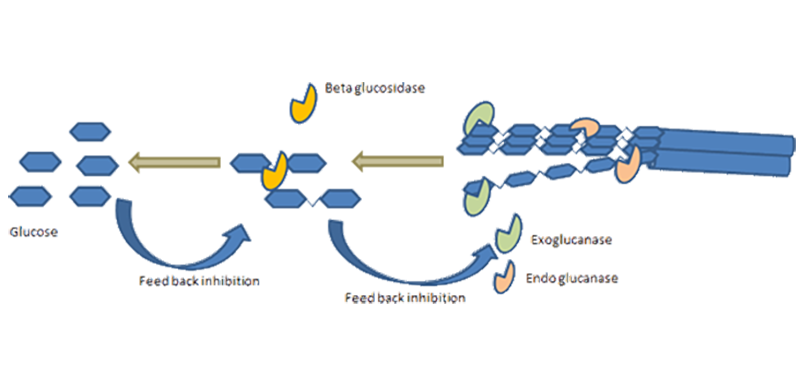
Hydrolysis
Enzymes
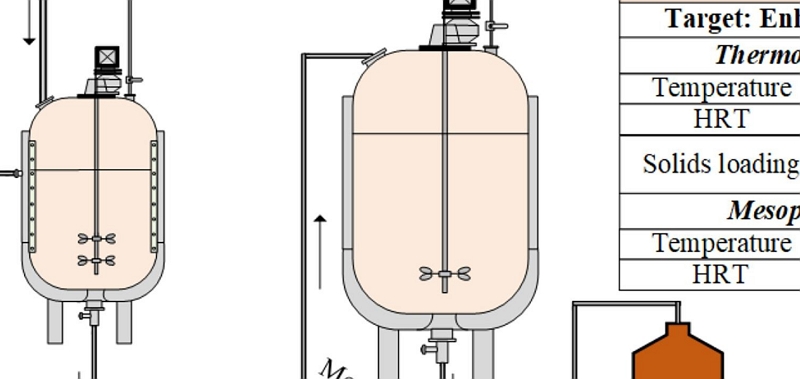
Downstream Processing
Lab-Scale Optimisations
TRL Scale-Up
Technoeconomic Analyses
Biobased Chemicals
From Process Refinements to an Entire New Process
Research Collaborations
Hydrothermal carbonization (HTC) research has mainly focused on primary char production, with limited attention to secondary char, which is formed through polymerization and condensation of dissolved organic compounds in the liquid phase. This research aims to address this gap via an experimental investigation of the impact of stirring on the mass and carbon balance of HTC reaction products, surface functional groups, and surface morphology of secondary char, using fructose as a model compound. A 3D hydrodynamic simulation model was developed for a two-liter HTC stirred reactor. The experimental results indicated that stirring did not significantly influence the pH, mass, carbon balance, and surface functional groups of secondary char produced under the range of experimental conditions (180 C, 10% biomass to water (B/W) ratio, and a residence time of 0-120 min) studied. Nonetheless, it was observed that a stirring rate of 200 rpm influenced the morphology and shape of the secondary char microspheres, leading to a significant increase in their size i.e., from 1-2 um in unstirred conditions compared with 70 um at a stirring rate of 200 rpm. This increase in size was attributed to the aggregation of microspheres into irregular aggregates at stirring rates > 65 rpm and residence times > 1 h. The hydrodynamic model revealed that high turbulence of Re > 104 and velocities > 0.17 m s-1 correlated with regions of secondary char formation, emphasizing their role in particle aggregation. Particle aggregation is significant above a stirring rate of 65 rpm, which corresponds to the onset of turbulent flow in the reactor. Finally, a mechanism is proposed, based on reactor hydrodynamics under stirred conditions, that explains secondary char deposition on the reactor walls and stirrer. | |
A dried dairy processing sludge (sludge from wastewater treatment of an effluent from a milk processing plant) was pyrolysed in a single-particle reactor at different temperatures from 400 C to 900 C. NH3 and HCN were measured online and offline by means of FTIR as well as by cumulative sampling in impinger bottles (in 0.05 M H2SO4 and 1 M NaOH, respectively) and analysed by photometric method. NO and NO2 were measured online using a nitric oxide analyser while N2O was measured by FTIR. Nitrogen (N) in the sludge and in the remaining char, char-N, was determined. Moreover, tar content in pyrolysis gas was measured and tar-N was determined. The results with respect to N mass balance closure are discussed. The different measurements techniques are compared. For pyrolysis at 520 and 700 nitrogen in the gas phase was mainly contained as N2 (36 % and 40 % respectively), followed by NH3 (15 % and 18 %), tar-N (10 % and 9 %), HCN (1 % and 3 %), NO (1 %) and NO2 (0.2 %). The dairy processing sludge has very specific properties with organic-N present predominantly as proteins and a high content of inherent Ca. These characteristics affected the distribution of N. The amount of char-N was higher while the amount of tar-N lower than for sewage sludge from literature, at comparable pyrolysis temperature. | |
Dairy processing sludge (DPS) is a byproduct generated in wastewater treatment plants located in dairy (milk) processing companies (waste activated sludge). DPS presents challenges in terms of its management (as biosolids) due to its high moisture content, prolonged storage required, uncontrolled nutrient loss and accumulation of certain substances in soil in the proximity of dairy companies. This study investigates the potential of hydrothermal carbonization (HTC) for recovery of nutrients in the form of solid hydrochar (biochar) produced from DPS originating from four different dairy processing companies. The HTC tests were carried out at 160 C, 180 C, 200 C and 220 C, and a residence time of 1h. The elemental properties of hydrochars (biochars), the content of primary and secondary nutrients, as well as contaminants were examined. The transformation of phosphorus in DPS during HTC was investigated. The fraction of plant available phosphorus was determined. The properties of hydrochar (biochar) were compared against the European Union Fertilizing Products Regulation. The findings of this study demonstrate that the content of nutrient in hydrochars (biochars) meet the requirements for organo-mineral fertilizer with nitrogen and phosphorus as the declared nutrients (13.9-26.7%). Further research on plant growth and field tests are needed to fully assess the agronomic potential of HTC hydrochar (biochar). | |
Disposal of waste-activated sludge [dairy processing sludge, (DPS)] from wastewater treatment plants located in milk processing companies is an increasing concern. DPS is usually applied to farmlands in the vicinity of the dairy companies. This practice is becoming unsustainable due to uncontrolled nutrient loss and potential soil contamination. We propose to recover nutrients in the form of biochar. This paper examines the properties of biochars obtained from slow pyrolysis of DPS. DPS samples were pyrolyzed at laboratory and pilot scale at 600 and 700 C. The elemental properties of biochars, the content of primary and secondary nutrients, as well as contaminants were examined and compared against the European Union Fertilizing Products Regulation. The biochars meet the specified limits for hydrogen-to-organic carbon ratio, chloride, and polycyclic aromatic hydrocarbons intended for gasification and pyrolysis component category materials. In six out of eight biochars, the content of phosphorus (P) as a single declared nutrient and the level of contaminants meet those required for an organo-mineral fertilizer. Only two biochars meet the required concentrations of nitrogen, phosphorus, and potassium. A minimum solid content of 30% in DPS is required to make the process of biochar production energetically sustainable. | |
Anaerobically digested sewage sludge mixed with forest residues was pyrolysed at 800 C, at laboratory and pilot scale. The study quantified differences in char and gas yields for tests carried out in a simple fixed bed laboratory reactor and rotating retort pyrolyser at pilot scale, when the residence time of feedstock was 10 min in both cases. The yield of char from pilot scale was 4 % lower than from laboratory scale while the yield of gas was 15.7 % higher. During the pilot scale pyrolysis of anaerobically digested sewage sludge blended with forest residues the gas quality for energy recovery applications was assessed and the fate of impurities (tar, NH3 and H2S) was investigated. The raw pyrolysis gas contained 14.6 g/Nm3 of tar, 36.9 g/Nm3 of NH3 and 793 ppm of H2S. Sixteen N-containing tar species were identified of which pyridine, propenenitrile, 2-methyl-, benzonitrile, and indole are found to be the most abundant. The yield of N-containing tar compounds accounted for approx. 12 % of total tar content. Conditioned pyrolysis gas contained 7.1 g/Nm3 of tar, 0.036 g/Nm3 of NH3 and 119 ppm of H2S. Benzene was by far the most abundant tar compound followed by toluene and styrene. The specifications of the used internal combustion engine were exceeded due to the sum of tar compounds such as fluorantrene and pyrene with 4+ aromatic rings (at 0.0015 g/Nm3) and NH3 content The effectiveness and sustainability of energy recovery in wastewater treatment can be improved using forest industry by-products. | |
Adsorption of six contaminants of emerging concern (CECs) - caffeine, chloramphenicol, carbamazepine, bisphenol A, diclofenac, and triclosan - from a multicomponent solution was studied using activated biochars obtained from three lignocellulosic feedstocks: wheat straw, softwood, and peach stones. Structural parameters related to the porosity and ash content of activated biochar and the hydrophobic properties of the CECs were found to influence the adsorption efficiency. For straw and softwood biochar, activation resulted in a more developed mesoporosity, whereas activation of peach stone biochar increased only the microporosity. The most hydrophilic CECs studied, caffeine and chloramphenicol, displayed the highest adsorption (22.8 and 11.3 mg g-1) onto activated wheat straw biochar which had the highest ash content of the studied adsorbents (20 wt%). Adsorption of bisphenol A and triclosan, both relatively hydrophobic substances, was highest (31.6 and 30.2 mg g-1) onto activated biochar from softwood, which displayed a well-developed mesoporosity and low ash content. | |
Magnetic carbons can significantly lower the costs of wastewater treatment due to easy separation of the adsorbent. However, current production techniques often involve the use of chlorinated or sulfonated Fe precursors with an inherent potential for secondary pollution. In this study, ochre, an iron-rich waste stream was investigated as a sustainable Fe source to produce magnetic activated biochar from two agricultural feedstocks, softwood and wheat straw. Fe doping resulted in significant shifts in pyrolysis yield distribution with increased gas yields (+50%) and gas energy content (+40%) lowering the energy costs for production. Physical activation transformed ochre to magnetite/maghemite resulting in activated magnetic biochars and led to a 4-fold increase in the adsorption capacities for two common micropollutants - caffeine and fluconazole. The results show that Fe doping not only benefits the adsorbent properties but also the production process, leading the way to sustainable carbon adsorbents. | |
The majority of the sludge from the treatment of wastewater in milk processing plants is land spread. The drawbacks of land spreading include local oversupply due to high transport costs, which results in sludge being spread on lands in the vicinity of the dairy factories. Local oversupply can lead to accumulation of certain substances in soil through annual application over many years. Therefore, in the long term, there is a need for alternative methods to recover energy and nutrients from increasing volumes of sludge generated from dairy processing. Pyrolysis offers a potential alternative to land spreading, which can reduce health and environmental risks, while providing an avenue for the recovery of energy and nutrients. Pyrolysis allows energy recovery in the form of a high calorific value pyrolysis gas and a char which may be used as a soil amendment. In this study pyrolysis of dried dairy sludge was carried out at pilot scale. The results indicate that a dried biological sludge can be successfully pyrolysed and when mixed with wood the resulting char meets European Biochar Certificate criteria regarding carbon content. Most of the initial energy content of the feedstock was retained in the pyrolysis gas prior to cleaning, 53%, compared to 34.5% in the char and 1.5% in the tar. For the pyrolysis gas after cleaning (mainly cracking in presence of air) the initial energy content of the feedstock retained in the gas was only slightly higher than that retained in the char, 39.2% versus 34.5%, while the tar accounted for 0.8% of the initial energy content. | |
Eucalypts can be very productive when intensively grown as short rotation woody crops (SRWC) for bioproducts. In Florida, USA, a fertilized, herbicided, and irrigated cultivar planted at 2471 trees/ha could produce over 58 green mt/ha/year in 3.7 years, and at 2071 trees/ha, its net present value (NPV) exceeded $750/ha at a 6% discount rate and stumpage price of $11.02/green mt. The same cultivar grown less intensively at three planting densities had the highest stand basal area at the highest density through 41 months, although individual tree diameter at breast height (DBH) was the smallest. In combination with an organic fertilizer, biochar improved soil properties, tree leaf nutrients, and tree growth within 11 months of application. Biochar produced from Eucalyptus and other species is a useful soil amendment that, especially in combination with an organic fertilizer, could improve soil physical and chemical properties and increase nutrient availability to enhance Eucalyptus tree nutrition and growth on soils. Eucalypts produce numerous naturally occurring bioproducts and are suitable feedstocks for many other biochemically or thermochemically derived bioproducts that could enhance the value of SRWCs. | |