Production of Biobutanol from Biomass
Biobased Chemicals - Background
Rationale for Biobased Chemicals Production
The production of chemicals from biomass, also known as bio-based chemicals, plays a critical role in creating a sustainable and environmentally friendly future, particularly as the world strives to reduce dependence on fossil fuels. Some of the advantages of biobased chemicals are listed below:- Climate Change Mitigation - Unlike fossil fuels, bio-based chemicals are made from plant materials that recently absorbed carbon dioxide from the atmosphere.
- Security of Supply - Fossil fuel supply often is reliant on geopolitically unstable regions. Developing domestic sources of biomass and the infrastructure to convert such feedstocks to chemicals, can help improve national chemicals security.
- Sustainable Development - Biomass can often be produced and processed locally, promoting rural development and creating jobs in agriculture, industry, and research.
- Waste Management - Biomass for bio-based chemicals can come from waste residues from agriculture, forestry, or even municipal waste. Using these waste streams for biobased chemicals can help solve waste disposal problems.
- Biodegradability - Many bio-based chemicals and the products made from them are biodegradable, avoiding environmental pollution associated with many fossil-derived resources.
- Resource Efficiency - The use of biomass as a raw material can contribute to a more circular economy, where waste from one process becomes the feedstock for another. This approach increases resource efficiency and reduces environmental impact compared to linear models of production.
Approaches for the Production of Biobased Chemicals
There are two main ways in which biobased chemicals can be obtained from biomass feedstocks:- Direct Extraction from Biomass - In this approach the target chemicals already exist within the feedstock. Hence, the focus of the bioprocess is on the extraction of the target chemical and then on subsequent separation and purification steps. CBD (cannabidiol), an alkaloid obtained from extracts of the hemp (cannabis) plant, is one example, among thousands, of a biobased chemical obtained this way.
- Production from Biomass or Biomass-Derived Compounds - Here the biobased chemical does not exist natively in the feedstock but is produced from it. This conversion can involve chemical, thermal, catalytic, and biological approaches or a combination of these. It is usually the case that the key stage of the bioprocess, where the biobased chemical is produced, works on a fraction, or derivative, of the original biomass feedstock. For example, ethanol can be produced via fermentation of the monomeric sugars obtained when the lignocellulosic polysaccharides (cellulose and/or hemicellulose) are hydrolysed. Alternatively, ethanol can also be produced via catalytic reforming of the syngas produced in the gasification of biomass.
How Celignis Can Help
At Celignis our multidisciplinary team has strong understanding of: biomass chemistry, bioprocessing technologies, and the mechanisms and challenges involved in producing a wide variety of biobased chemicals. We are ready to work with you on developing a suitable bioprocess to either obtain your targeted biobased chemical from biomass or to obtain the most appropriate biobased chemicals from a given feedstock.
Butanol Chemistry and Applications
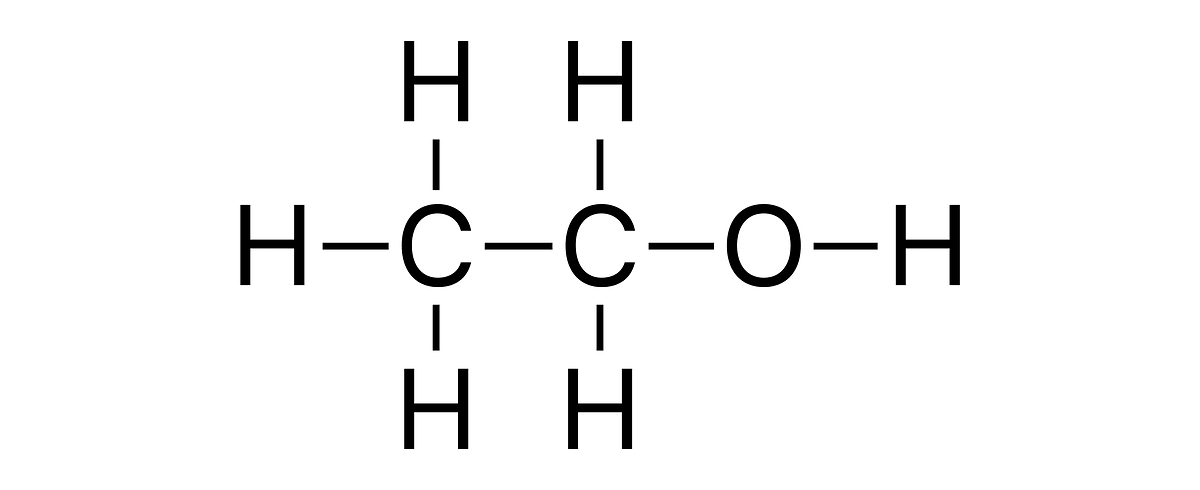
Butanol, also known as butyl alcohol, is a four-carbon alcohol (C4H9OH) with a molecular structure that gives it properties midway between those of ethanol (a two-carbon alcohol) and gasoline (typically composed of eight to twelve carbon hydrocarbons). There are four isomeric structures for butanol: n-butanol, sec-butanol, tert-butanol, and isobutanol, each having different properties and applications.Biobutanol refers to biobased butanol, i.e. that is produced from renewable biomass sources, rather than from petroleum. This production is generally achieved via a microbial fermentation process, often using species of bacteria from the Clostridium genus.
Biobutanol has many important applications, a few of these are listed below:
- Biofuel - Butanol's energy content is closer to that of gasoline than ethanol, and it is less hygroscopic and corrosive, making it better suited for use in internal combustion engines and for blending with gasoline. Furthermore, butanol can be transported through existing fuel pipelines, unlike ethanol.
- Chemical Feedstock - Butanol is a valuable feedstock for the production of a wide range of chemicals. For instance, it can be used in the manufacture of butyl acrylate, which is used in the production of acrylics. Other applications include the production of plastics, synthetic rubber, and solvents.
History of Butanol Production
The production of butanol has switched between being largely biobased to primarily fossil fuel-derived and is now moving back towards a biobased approach due to sustainability and environmental concerns.The production of biobutanol started in the early 1900s. The Chaim Weizmann process used Clostridium bacteria to ferment sugar into a mixture of acetone, butanol, and ethanol (ABE fermentation). This process became widely known as the Weizmann fermentation or ABE process. The primary driver for this technology was the need for acetone, which was used as a solvent in the manufacture of cordite, a smokeless propellant needed in large quantities during World War I. The ABE fermentation process was commercially successful during the first half of the 20th century. By the 1950s, the production of biobutanol from sugar and starch feedstocks was a large-scale industry.
However, the discovery of new oil fields, development of petroleum refining technology, and the invention of catalytic processes in the mid-20th century led to a shift from biobased butanol to fossil fuel-derived butanol. It became cheaper and more efficient to produce butanol from propylene, a product of petroleum refining, than to ferment biomass. One commonly used process is the oxo process (also known as hydroformylation), which involves the reaction of propylene with synthesis gas (a mixture of carbon monoxide and hydrogen) to produce butyraldehyde, which is then hydrogenated to produce butanol. This method of production became dominant due to its lower cost, high efficiency, and the growing availability of petroleum feedstocks.
In recent years, rising concerns over fossil fuel depletion, climate change, and the desire for energy security sparked renewed interest in biobased butanol production. Research efforts have been focused on improving the ABE fermentation process and developing new biological and chemical processes for converting biomass into butanol.
ABE (Acetone-Butanol-Ethanol) Fermentation
ABE fermentation is the conventional and most widely practiced method for biobutanol production. It uses Clostridium bacteria to ferment a variety of sugars into a mixture of acetone, butanol, and ethanol.However, there are several challenges with ABE fermentation:
- Product Inhibition - The biggest challenge in ABE fermentation is product inhibition. Butanol is toxic to the Clostridium bacteria, which reduces their productivity and ultimately limits the butanol concentration in the broth to about 2% by volume. This low concentration increases the energy needed for product recovery.
- Low Yield and Productivity - The overall yield and productivity of ABE fermentation are relatively low compared to other biofuels, such as ethanol. This is mainly due to the complex metabolism of the Clostridium bacteria and the partitioning of the carbon flux among acetone, butanol, and ethanol.
- Substrate Flexibility - Although Clostridium bacteria can utilize a wide range of sugars, their ability to ferment pentose sugars (such as xylose, a major component of lignocellulosic biomass) is relatively poor. This limits the types of feedstocks that can be used in ABE fermentation.
- Metabolic Engineering - Genetic engineering techniques can modify the metabolic pathways of Clostridium bacteria to increase butanol production and reduce the production of other compounds, such as acetone.
- Product Recovery - Techniques to continuously remove butanol from the fermentation broth can be employed. This helps to alleviate product inhibition and improve process efficiency. Examples include gas stripping, pervaporation, and adsorption.
- Co-culture Systems - In co-culture systems different microbial species are used together to complement each other's metabolic capabilities. For example, a second organism could be used to consume butanol, alleviating product inhibition for Clostridium.
- Pentose Fermentation - Efforts are being made to engineer Clostridium strains that can effectively ferment pentose sugars, which would expand the range of feedstocks that can be used in ABE fermentation.
BE (Butyrate-Ethanol) Fermentation
BE (Butyrate-Ethanol) fermentation is an alternative to the traditional Acetone-Butanol-Ethanol (ABE) fermentation process and is designed to overcome some of the challenges associated with ABE fermentation. BE fermentation is essentially a metabolic engineering strategy aimed at producing more butanol and less of the other by-products such as acetone.The concept of BE fermentation involves modifying the metabolic pathway in Clostridium bacteria to reduce acetone formation and enhance butanol and ethanol production. Butanol and ethanol, being the more valuable products, are preferred in higher concentrations, and so a metabolic pathway favoring these two products would be beneficial.
However, there are still several challenges with BE fermentation:
- Metabolic Balance - Creating a strain of bacteria that produces more butanol and less acetone requires a careful balance of metabolic activities. It is not a straightforward process and often involves complex genetic engineering.
- Product Toxicity - Like ABE fermentation, BE fermentation also suffers from the problem of butanol toxicity. Butanol inhibits bacterial growth and fermentation activity, so a high butanol concentration is still problematic. Hence, similar to ABE fermentation, strategies for in-situ product recovery such as gas stripping, adsorption, and pervaporation can be applied in BE fermentation to alleviate butanol toxicity.
- Increased Butanol Production - By altering the metabolic pathway to favor butanol and ethanol production, the BE process can potentially produce more butanol than the ABE process.
- Reduced Byproducts - BE reduces the production of acetone, which is less valuable and can interfere with butanol recovery.
- Technical Complexity - Engineering a microbial strain that consistently produces a high ratio of butanol to other products can be technically challenging and requires precise control over the microbial metabolism.
- Product Toxicity - Despite the shift in metabolic activity, the problem of butanol toxicity remains, and this still needs to be addressed to achieve an efficient and viable process.
Biomass Hydrolysis
The fermentation of biobutanol requires sugars. The easiest way to obtain such sugars are from what are termed first-generation feedstocks. These are crops (e.g. sugarcane, wheat, corn) that contain these sugars in forms which are easy to hydrolyse. There are well-established efficient routes for hydrolysing sucrose and starch to monomeric glucose. However, 1st generation feedstocks can be costly, require large energy and chemical inputs for their production, and their use for biofuels and biobased chemicals can conflict with their use for food.Much of the current focus of bioprocess development on biobutanol production concerns utilising lignocellulosic feedstocks. These feedstocks contain sugars, like many 1st generation feedstocks, that can be used as substrates for the production of biobutanol via fermentation. However, the difference is that these sugars are part of the structural polysaccharides cellulose and hemicellulose, rather than the easier to process sucrose and starch.
In the case of cellulose, the challenge is in effectively hydrolysing it to obtain monomeric glucose. This hydrolysis can use acid but generally involves the action of cellulase enzymes. It is more challenging to hydrolyse cellulose than starch due to the crystalline nature of cellulose and also due to complications associated with the wider lignocellulosic matrix. For example, the activity of cellulase enzymes is often hindered in many lignocellulosic feedstocks due to the presence of lignin. As a result, most bioprocesses require a pretreatment stage that make the substrate more amenable for enzymatic hydrolysis.
Challenges in Fermenting Hydrolysates of Lignocellulosic Biomass
The pretreatment and hydrolysis stages may also release other compounds into the liquid phase, for example components of the extractives, some sugar degradation products (e.g. furans, such as furfural, and organic acids), and phenolics. These compounds can complicate the downstream fermentation, hence it is important that either robust microorganisms are used for the fermentation or that the concentrations of such fermentation inhibitors are minimised. Achieving these aims requires careful work in the bioprocess development.Additionally, if fermentation of non-cellulosic sugars (i.e. hemicellulose sugars) to biobutanol is also targeted then this requires additional work in developing this process. This is because, while the Clostridium bacteria (typically used in butanol fermentation) can ferment hexose sugars (such as glucose) well, they are less efficient at fermenting pentose sugars (such as xylose), which are a significant part of hemicellulose.
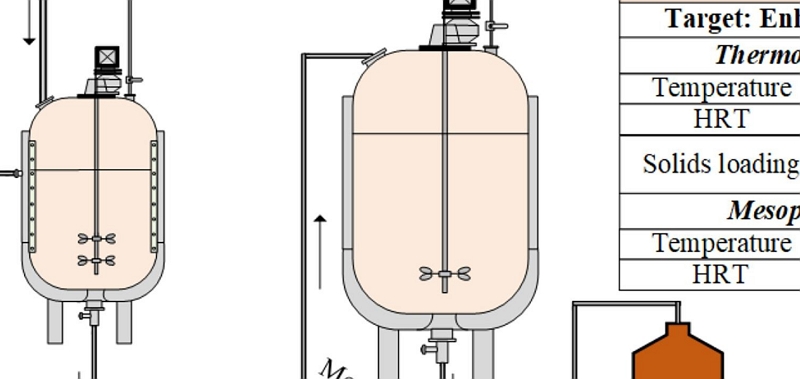
There are several approaches that can be used for the hydrolysis of lignocellulosic feedstocks and the subsequent fermentation of the liberated sugars to biobutanol. These include: 1. Separate hydrolysis and fermentation (SHF); 2. Simultaneous saccharification and fermentation (SSF); 3. Simultaneous saccharification and co-fermentation (SSCF). SHF allows both enzymes and microbes to operate at their optimum conditions, but is limited with product inhibition to enzymes and feed inhibition to microbes. This is overcome in SSF and SSFC fermentation where the enzymatic product glucose is consumed by microbes as it is produced. High-solids-loading fermentation with butanol-tolerant microbial strains allows production of high concentrations of butanol. However, high-solids fermentation causes viscosity issues and hence suitable fermentation regimes and mixers should be designed in order to obtain maximum butanol concentrations, yields and productivity.
Click here to read more about biobutanol fermentation from lignocellulose.
Get more info...Biobutanol Fermentation
Valorisation of Other Biomass Components
Lignocellulosic biomass feedstocks vary greatly in their compositions, however they all contain cellulose, hemicellulose, and lignin. In addition, some lignocellulosic biomass can contain significant amounts of extractives as well as ash and protein.The production of second-generation biobutanol is complex and requires significant CAPEX and OPEX. In the case of a bioprocess that is only focused on the production of biobutanol from the cellulose fraction, 60+% of the biomass (depending on composition) may not be part of the primary conversion mechanism. The viability of the bioprocess can improve if greater proportions of the biomass are valorised. The ways this can happen will depend on the pretreatment, hydrolysis, and fermentation technologies being employed.
For example, an organosolv pretreatment may remove the lignin and hemicellulose into the liquid phase, leaving the cellulose in the residual solid that is then hydrolysed to glucose and fermented to biobutanol. Here, there may be opportunites to separately valorise the lignin and hemicellulose fractions, allowing for potentially enhanced revenue from the feedstock but leading to a more complex process as these downstream treatments need to be integrated. Conversely, a steam explosion pretreatment would physically-disrupt the biomass but leave much more of the hemicellulose and lignin in the cellulose-containing solid. In this scenario, cellulose and hemicellulose could both be hydrolysed and the liberated sugars fermented to biobutanol. This could potentially lead to higher biobutanol yields compared to the first example, where only cellulose was hydrolysed. The post-hydrolysis solids would contain much of the lignin, as well as unhydrolysed portions of cellulose and hemicellulose. This substrate would not have the potential value of the isolated lignin fraction from the organosolv pretreatment but it could be combusted to provided energy for the bioprocess.
The choice of how different fractions of biomass are valorised is a key part of any bioprocess and requires detailed understanding of lignocellulose chemistry, conversion mechanisms, and product markets. At Celignis our multidisciplinary team are experts in all these sectors and can work with you on developing an optimised biobutanol production bioprocess that effectively valorises the feedstock.
Gasification
An alternative route for producing biobutanol from lignocellulosic feedstocks is via the catalytic or biological reforming of the gases produced from the thermal processing of the biomass, with gasification considered to be the most suitable thermal treatment.Gasification targets the production of a synthesis gas, also known as syngas. This gas is a mixture of mainly carbon monoxide (CO) and hydrogen (H2). The gasification process typically involves high temperatures (above 800oC) in the presence of a controlled amount of oxygen or steam. Since the formation of the gas from gasification is endothermic, the necessary temperature is attained via the oxygen-burning of a portion of the feedstock. When air is used to supply the oxygen the resulting gas is termed a producer gas. However, the use of air is only a viable option for gasification technologies where electricity production is the target. The catalytic processes required for the synthesis of biobutanol require a much cleaner gas.
The general formula for the oxygen-fuelled gasification of carbohydrates is included below:
C6H12O6 + 3⁄2O2 -> 6CO + 3H2 + 3H2O
The ratio of hydrogen to carbon-monoxide in the syngas can be adjusted, according to downstream biobutanol production requirements, using the Water-Gas Shift (WGS) reaction. This involves carbon monoxide and water, producing carbon dioxide and hydrogen:
CO + H2O -> CO2 + H2
There are two main types of WGS reactions: high-temperature WGS (HTWGS) and low-temperature WGS (LTWGS). HTWGS operates at around 350-450oC and is typically the first stage due to its greater conversion efficiency. LTWGS operates at around 200-250oC and helps to convert any remaining CO.
Syngas to Biobutanol (Catalytic Approach)
The conversion of syngas to biobutanol is a thermochemical process typically carried out using a heterogeneous catalyst. The catalytic conversion process involves a series of complex reactions, with two key steps: the formation of intermediate compounds (such as aldehydes or alcohols) through a process known as Fischer-Tropsch synthesis, followed by the conversion of these intermediates into butanol.Fischer-Tropsch Synthesis: The Fischer-Tropsch (FT) process is well-established and has been used for many years to produce liquid fuels and chemicals from syngas. However, there can be complications with this route when using syngas derived from biomass feedstocks. The process involves reactions between carbon monoxide and hydrogen on the surface of a metal catalyst (commonly iron or cobalt) to produce a range of hydrocarbons. Adjusting the FT process conditions can shift the product distribution towards oxygenates like alcohols and aldehydes, which can then be converted into butanol.
Alcohol Formation: The next step involves further reaction of these intermediate compounds to produce butanol. This can involve a range of reactions, including aldol condensation, hydrogenation, and esterification.
The design of the catalyst plays a key role in this process. It should be able to facilitate both the FT synthesis and the subsequent conversion steps while minimizing unwanted side reactions. Challenges associated with the catalytic conversion of syngas to butanol include:
- Catalyst Selectivity and Stability - Developing a catalyst that selectively produces butanol over other possible products (like methane, methanol, or longer-chain hydrocarbons) is challenging. Additionally, the catalyst should be stable under the harsh reaction conditions and over long operating times.
- Reaction Conditions - The process must be conducted under specific temperatures, pressures, and syngas compositions to optimize butanol production. Balancing these conditions to maximize yield and selectivity while minimizing energy input and equipment costs can be challenging.
- Product Separation - Butanol produced during the synthesis process must be separated and purified from the product stream, which can be energy-intensive and complicated due to the presence of a variety of other products and unreacted syngas.
Syngas Fermentation
Syngas fermentation, also known as gas fermentation or syngas bioconversion, is an alternative, biological, route for the production of products (such as biobutanol) from syngas. The process employs a group of microorganisms known as acetogens, a type of anaerobic bacteria capable of using carbon monoxide (CO), carbon dioxide (CO2), and hydrogen (H2) - the primary constituents of syngas - to grow and produce various chemicals. These organisms follow a metabolic pathway known as the Wood-Ljungdahl pathway or Acetyl-CoA pathway.In the case of butanol production, the formation of butyrate from acetyl-CoA involves a series of reactions including condensation, reduction, and esterification steps. Butyrate can then be converted to butanol via a two-step reduction process.
Certain species of Clostridium, like Clostridium autoethanogenum and Clostridium ljungdahlii, are well-known for their ability to consume syngas and produce bioethanol and acetate as their primary products. Butanol production from these strains is less common and typically lower yield, but research is ongoing to enhance butanol production, such as through metabolic engineering and process optimization. Some species of Acetobacterium, like Acetobacterium woodii, can also metabolize syngas, although they are less commonly used in industrial processes compared to Clostridium.
Syngas fermentation has several potential advantages over the catalytic route. It can operate at relatively low temperatures and pressures, reducing energy costs, and it can also potentially handle a wider range of syngas compositions, since the bacteria can adjust their metabolism to use available resources. Additionally, it has the potential to produce a range of other valuable chemicals in addition to biobutanol.
However, syngas fermentation also presents some challenges:
- Slow Growth Rates and Productivity - The bacteria used in syngas fermentation typically have slow growth rates and relatively low product yields and productivity compared to traditional sugar fermentation processes.
- Gas-Liquid Mass Transfer - Efficient transfer of the gaseous substrates (CO, CO2, H2) into the liquid phase where the bacteria reside is technically challenging but crucial for process efficiency.
- Inhibitory Compounds - The syngas may contain impurities like sulphur compounds, nitrogen compounds, or particulates, depending on the gasification process and feedstock used. These impurities can inhibit the growth of the bacteria and reduce the process efficiency.
- Product Separation - Butanol is toxic to many microorganisms at higher concentrations, and its recovery from the fermentation broth can be challenging due to its relatively high boiling point and affinity for water.
Syngas Butanol vs. Hydrolysis Butanol
Advantages of biobutanol production via gasification:- Conversion of Most of the Biomass - Any biomass component containing carbon and hydrogen atoms can contribute to gasification syngas, whilst when the hydrolysis platform is employed only those fractions containing carbohydrates (i.e. cellulose and hemicellulose) can contribute towards the production of biobutanol.
- Feedstock Flexibility - Related to the above, gasification can handle a wide variety of feedstocks, including mixed and low-quality feedstocks, due to its high processing temperature and capacity to convert all volatile components of biomass into syngas.
- Minimal Pretreatments Required - Unlike the hydrolysis platform, gasification requires minimal sample pretreatment, with particle-size reduction (mechanical pretreatment) usually being the only pretreatment employed.
- Gas Cleanup - The raw syngas from gasification contains various contaminants (tars, particulates, sulphur and nitrogen compounds) that must be effectively cleaned before the subsequent conversion process, which adds complexity and cost to the process.
- Catalyst Sensitivity - In catalytic conversion, the catalysts used for syngas to butanol conversion can be sensitive to impurities in the syngas and may deactivate over time, which can affect process efficiency and economics.
- Process Conditions - Gasification requires high temperatures and pressures, which can increase operational challenges and costs.
- Uncompetitive at Small Scales - The technologies employed for gasification, syngas cleanup, and catalytic/biological reforming of the syngas to butanol are relatively new and complex. As a result, they currently need to operate at large scales in order for the process to be economically viable. This places a high CAPEX barrier to entry.
1. Understanding Your Requirements
Prior to undertaking bioprocess projects we learn from our clients what their targets are from the process as well as whether there are any restrictions or requirements that may need to form the boundaries of the work that we undertake. These help to guide us to then prepare a potential bioprocess development project.
For example, in the context of biobutanol production, the primary focus of one client may be on the fermentation of cellulose-derived glucose to butanol whilst another client may be satisfied with lower butanol yields from cellulose providing that the hemicellulose fraction can also be used for biobutanol production. These different preferences are likely to influence our choices of pretreatment technology, hydrolysis and fermentation approaches, and process conditions.
2. Detailed Feedstock Analysis
In cases where you have already selected a feedstock for the bioprocess, we would then undertake a detailed compositional analysis (P10 or, ideally, P19) of representative samples of that feedstock.
In cases where the feedstock has not yet been selected we can review your list of candidate feedstocks, selecting top candidates based on our prior experience in their analysis and bioprocessing. If you do not have a list of candidate feedstocks then we can provide one, based on your location and the requirements outlined in Stage 1. We would then analyse in detail these priority feedstocks and come to a decision, based on the compositional data and other relevant factors (e.g. price, supply, consistency etc.) on a selected feedstock for the project.
At this point of the project, the Celignis Bioprocess team typically meet to discuss and prepare a project proposal for the development of a bioprocess for biobutanol production from this feedstock. After this proposal is reviewed by the client, and revised if needed, we are then ready to start work on the next stages.
3. Pretreatment Optimisation (Lab-Scale)
Our projects usually involve undertaking a number of pretreatment experiments, covering a variety of process conditions. We follow a scientifically-based Design of Experiments (DoE) protocol where the criteria and boundaries for this DoE are formulated in close collaboration with our clients, considering the chemistry of the feedstock(s) and our understandings of the mechanisms of biomass pretreatment.
We usually recommend that these initial optimisation experiments are undertaken at the lab-scale (around TRL3) in order to reduce costs and the length of the project. For each experiment we analyse the solid and liquid outputs of the pretreatment process, leading to a detailed data-set where effects of process conditions on the yield and composition of the various streams can be explored and mapped.
We can also undertake a second iteration of lab-scale experiments in order to fine-tune the conditions based on the knowledge gained in the initial experiments.
4. Hydrolysis & Fermentation Optimisation
At the project proposal stage we will have decided with regards to which hydrolysis/fermentation approach will be undertaken (i.e. Separate Hydrolysis and Fermentation (SHF); Simultaneous Saccharification and Fermentation (SSF); or Simultaneous Saccharification and Co-Fermentation (SSCF)).
Each of these approaches involves a number of different process conditions (e.g. solid-loading, temperature, reaction times, mixing) that need to be optimised. Hence, this stage of the project involves a similar DoE being undertaken where these conditions are narrowed down. Again, these optimisation experiments are undertaken at the lab-scale in order to accelerate the outputs and reduce project costs.
This stage usually follows the pretreatment optimisation activities, however it is possible that there can be some overlap in order to reach the final project outputs more quickly.
5. Biobutanol Recovery
Based on the outputs of the prior lab-scale Stages we can optimise the methods employed for separating and purifying the biobutanol from the fermentation beer. We can also potentially look at the recovery of other compounds from the broth.
It is possible for this Stage to run alongside Stage 4.
6. Valorisation of Remaining Biomass
Depending on the pretreatment, hydrolysis, and fermentation processess employed in the project, we can look at valorising those biomass components that are not hydrolysed to sugars and fermented to biobutanol. For example, we can analyse the enzymatic hydrolysis residue for its suitability for combustion. In another scenario we can evaluate the potential for valorising the hemicellulose sugars that are hydrolysed in the dilute-acid-pretreatment stage.
7. Validation at Higher TRLs
Once we have concluded our optimisation of the biobutanol bioprocess conditions at the lab-scale we can then test those conditions at higher technology readiness levels (TRLs). The scales at which we can operate are dependent on the type of technology employed, but can reach up to 100 litres.
We have all of the necessary downstream equipment to efficiently handle the solid and liquid streams arising from these scaled-up activities.
If we find that there are differences between the yield and compositions of the different streams, compared with our lab-scale experiments, then we can explore the potential reasons for these and work on final tweaks to optimise the bioprocess for higher TRLs.
8. Technoeconomic Analysis (TEA)
The Celignis team, including Oscar our chief TEA expert, can undertake a detailed technoeconomic analysis of the developed process. We apply accurate and realistic costing models to determine the CAPEX and OPEX of simulated and pilot scale processes which are then used to determine key economic indicators such as IRR, NPV and payback periods.
Within these TEAs we can undertake sensitivity analyses to assess the effect of variable costs and revenues on the commercial viability of the biobutanol production process.
Our preferred approach is to include TEA studies at each stage of the development of the bioprocess, so that the process can be optimised in a commercially-relevant way, followed by a more detailed TEA after the process has been optimised and tested at higher TRL levels.
Click here to read more about the technoeconomic analysis (TEA) services offered by Celignis.

Lalitha Gottumukkala
Founder of Celignis Bioprocess, CIO of Celignis
PhD
<p style="text-align: left;">Has a deep understanding of all biological and chemical aspects of bioproceses. Has developed Celignis into a renowned provider of bioprocess development services to a global network of clients.</p>

Oscar Bedzo
Bioprocess Project Manager & Technoeconomic Analysis Lead
PhD
<p style="text-align: left;">A dynamic, purpose-driven chemical engineer with expertise in bioprocess development, process design, simulation and techno-economic analysis over several years in the bioeconomy sector.</p>

Dan Hayes
Celignis CEO And Founder
PhD (Analytical Chemistry)
<p style="text-align: left;">Dreamer and achiever. Took Celignis from a concept in a research project to being the bioeconomy's premier provider of analytical and bioprocessing expertise.</p>
Bioethanol
Xylitol
Hydrothermal carbonization (HTC) research has mainly focused on primary char production, with limited attention to secondary char, which is formed through polymerization and condensation of dissolved organic compounds in the liquid phase. This research aims to address this gap via an experimental investigation of the impact of stirring on the mass and carbon balance of HTC reaction products, surface functional groups, and surface morphology of secondary char, using fructose as a model compound. A 3D hydrodynamic simulation model was developed for a two-liter HTC stirred reactor. The experimental results indicated that stirring did not significantly influence the pH, mass, carbon balance, and surface functional groups of secondary char produced under the range of experimental conditions (180 C, 10% biomass to water (B/W) ratio, and a residence time of 0-120 min) studied. Nonetheless, it was observed that a stirring rate of 200 rpm influenced the morphology and shape of the secondary char microspheres, leading to a significant increase in their size i.e., from 1-2 um in unstirred conditions compared with 70 um at a stirring rate of 200 rpm. This increase in size was attributed to the aggregation of microspheres into irregular aggregates at stirring rates > 65 rpm and residence times > 1 h. The hydrodynamic model revealed that high turbulence of Re > 104 and velocities > 0.17 m s-1 correlated with regions of secondary char formation, emphasizing their role in particle aggregation. Particle aggregation is significant above a stirring rate of 65 rpm, which corresponds to the onset of turbulent flow in the reactor. Finally, a mechanism is proposed, based on reactor hydrodynamics under stirred conditions, that explains secondary char deposition on the reactor walls and stirrer. | |
A dried dairy processing sludge (sludge from wastewater treatment of an effluent from a milk processing plant) was pyrolysed in a single-particle reactor at different temperatures from 400 C to 900 C. NH3 and HCN were measured online and offline by means of FTIR as well as by cumulative sampling in impinger bottles (in 0.05 M H2SO4 and 1 M NaOH, respectively) and analysed by photometric method. NO and NO2 were measured online using a nitric oxide analyser while N2O was measured by FTIR. Nitrogen (N) in the sludge and in the remaining char, char-N, was determined. Moreover, tar content in pyrolysis gas was measured and tar-N was determined. The results with respect to N mass balance closure are discussed. The different measurements techniques are compared. For pyrolysis at 520 and 700 nitrogen in the gas phase was mainly contained as N2 (36 % and 40 % respectively), followed by NH3 (15 % and 18 %), tar-N (10 % and 9 %), HCN (1 % and 3 %), NO (1 %) and NO2 (0.2 %). The dairy processing sludge has very specific properties with organic-N present predominantly as proteins and a high content of inherent Ca. These characteristics affected the distribution of N. The amount of char-N was higher while the amount of tar-N lower than for sewage sludge from literature, at comparable pyrolysis temperature. | |
Dairy processing sludge (DPS) is a byproduct generated in wastewater treatment plants located in dairy (milk) processing companies (waste activated sludge). DPS presents challenges in terms of its management (as biosolids) due to its high moisture content, prolonged storage required, uncontrolled nutrient loss and accumulation of certain substances in soil in the proximity of dairy companies. This study investigates the potential of hydrothermal carbonization (HTC) for recovery of nutrients in the form of solid hydrochar (biochar) produced from DPS originating from four different dairy processing companies. The HTC tests were carried out at 160 C, 180 C, 200 C and 220 C, and a residence time of 1h. The elemental properties of hydrochars (biochars), the content of primary and secondary nutrients, as well as contaminants were examined. The transformation of phosphorus in DPS during HTC was investigated. The fraction of plant available phosphorus was determined. The properties of hydrochar (biochar) were compared against the European Union Fertilizing Products Regulation. The findings of this study demonstrate that the content of nutrient in hydrochars (biochars) meet the requirements for organo-mineral fertilizer with nitrogen and phosphorus as the declared nutrients (13.9-26.7%). Further research on plant growth and field tests are needed to fully assess the agronomic potential of HTC hydrochar (biochar). | |
Disposal of waste-activated sludge [dairy processing sludge, (DPS)] from wastewater treatment plants located in milk processing companies is an increasing concern. DPS is usually applied to farmlands in the vicinity of the dairy companies. This practice is becoming unsustainable due to uncontrolled nutrient loss and potential soil contamination. We propose to recover nutrients in the form of biochar. This paper examines the properties of biochars obtained from slow pyrolysis of DPS. DPS samples were pyrolyzed at laboratory and pilot scale at 600 and 700 C. The elemental properties of biochars, the content of primary and secondary nutrients, as well as contaminants were examined and compared against the European Union Fertilizing Products Regulation. The biochars meet the specified limits for hydrogen-to-organic carbon ratio, chloride, and polycyclic aromatic hydrocarbons intended for gasification and pyrolysis component category materials. In six out of eight biochars, the content of phosphorus (P) as a single declared nutrient and the level of contaminants meet those required for an organo-mineral fertilizer. Only two biochars meet the required concentrations of nitrogen, phosphorus, and potassium. A minimum solid content of 30% in DPS is required to make the process of biochar production energetically sustainable. | |
Anaerobically digested sewage sludge mixed with forest residues was pyrolysed at 800 C, at laboratory and pilot scale. The study quantified differences in char and gas yields for tests carried out in a simple fixed bed laboratory reactor and rotating retort pyrolyser at pilot scale, when the residence time of feedstock was 10 min in both cases. The yield of char from pilot scale was 4 % lower than from laboratory scale while the yield of gas was 15.7 % higher. During the pilot scale pyrolysis of anaerobically digested sewage sludge blended with forest residues the gas quality for energy recovery applications was assessed and the fate of impurities (tar, NH3 and H2S) was investigated. The raw pyrolysis gas contained 14.6 g/Nm3 of tar, 36.9 g/Nm3 of NH3 and 793 ppm of H2S. Sixteen N-containing tar species were identified of which pyridine, propenenitrile, 2-methyl-, benzonitrile, and indole are found to be the most abundant. The yield of N-containing tar compounds accounted for approx. 12 % of total tar content. Conditioned pyrolysis gas contained 7.1 g/Nm3 of tar, 0.036 g/Nm3 of NH3 and 119 ppm of H2S. Benzene was by far the most abundant tar compound followed by toluene and styrene. The specifications of the used internal combustion engine were exceeded due to the sum of tar compounds such as fluorantrene and pyrene with 4+ aromatic rings (at 0.0015 g/Nm3) and NH3 content The effectiveness and sustainability of energy recovery in wastewater treatment can be improved using forest industry by-products. | |
Adsorption of six contaminants of emerging concern (CECs) - caffeine, chloramphenicol, carbamazepine, bisphenol A, diclofenac, and triclosan - from a multicomponent solution was studied using activated biochars obtained from three lignocellulosic feedstocks: wheat straw, softwood, and peach stones. Structural parameters related to the porosity and ash content of activated biochar and the hydrophobic properties of the CECs were found to influence the adsorption efficiency. For straw and softwood biochar, activation resulted in a more developed mesoporosity, whereas activation of peach stone biochar increased only the microporosity. The most hydrophilic CECs studied, caffeine and chloramphenicol, displayed the highest adsorption (22.8 and 11.3 mg g-1) onto activated wheat straw biochar which had the highest ash content of the studied adsorbents (20 wt%). Adsorption of bisphenol A and triclosan, both relatively hydrophobic substances, was highest (31.6 and 30.2 mg g-1) onto activated biochar from softwood, which displayed a well-developed mesoporosity and low ash content. | |
Magnetic carbons can significantly lower the costs of wastewater treatment due to easy separation of the adsorbent. However, current production techniques often involve the use of chlorinated or sulfonated Fe precursors with an inherent potential for secondary pollution. In this study, ochre, an iron-rich waste stream was investigated as a sustainable Fe source to produce magnetic activated biochar from two agricultural feedstocks, softwood and wheat straw. Fe doping resulted in significant shifts in pyrolysis yield distribution with increased gas yields (+50%) and gas energy content (+40%) lowering the energy costs for production. Physical activation transformed ochre to magnetite/maghemite resulting in activated magnetic biochars and led to a 4-fold increase in the adsorption capacities for two common micropollutants - caffeine and fluconazole. The results show that Fe doping not only benefits the adsorbent properties but also the production process, leading the way to sustainable carbon adsorbents. | |
The majority of the sludge from the treatment of wastewater in milk processing plants is land spread. The drawbacks of land spreading include local oversupply due to high transport costs, which results in sludge being spread on lands in the vicinity of the dairy factories. Local oversupply can lead to accumulation of certain substances in soil through annual application over many years. Therefore, in the long term, there is a need for alternative methods to recover energy and nutrients from increasing volumes of sludge generated from dairy processing. Pyrolysis offers a potential alternative to land spreading, which can reduce health and environmental risks, while providing an avenue for the recovery of energy and nutrients. Pyrolysis allows energy recovery in the form of a high calorific value pyrolysis gas and a char which may be used as a soil amendment. In this study pyrolysis of dried dairy sludge was carried out at pilot scale. The results indicate that a dried biological sludge can be successfully pyrolysed and when mixed with wood the resulting char meets European Biochar Certificate criteria regarding carbon content. Most of the initial energy content of the feedstock was retained in the pyrolysis gas prior to cleaning, 53%, compared to 34.5% in the char and 1.5% in the tar. For the pyrolysis gas after cleaning (mainly cracking in presence of air) the initial energy content of the feedstock retained in the gas was only slightly higher than that retained in the char, 39.2% versus 34.5%, while the tar accounted for 0.8% of the initial energy content. | |
Eucalypts can be very productive when intensively grown as short rotation woody crops (SRWC) for bioproducts. In Florida, USA, a fertilized, herbicided, and irrigated cultivar planted at 2471 trees/ha could produce over 58 green mt/ha/year in 3.7 years, and at 2071 trees/ha, its net present value (NPV) exceeded $750/ha at a 6% discount rate and stumpage price of $11.02/green mt. The same cultivar grown less intensively at three planting densities had the highest stand basal area at the highest density through 41 months, although individual tree diameter at breast height (DBH) was the smallest. In combination with an organic fertilizer, biochar improved soil properties, tree leaf nutrients, and tree growth within 11 months of application. Biochar produced from Eucalyptus and other species is a useful soil amendment that, especially in combination with an organic fertilizer, could improve soil physical and chemical properties and increase nutrient availability to enhance Eucalyptus tree nutrition and growth on soils. Eucalypts produce numerous naturally occurring bioproducts and are suitable feedstocks for many other biochemically or thermochemically derived bioproducts that could enhance the value of SRWCs. | |