Biorefining for Advanced Biofuels
Background
There are two major pathways by which second generation biofuels biorefineries operate: through hydrolysis processes that aim to liberate sugars from the lignocellulosic polysaccharides (i.e. cellulose and hemicellulose), and through thermochemical processes that degrade more extensively the components of both polysaccharides and lignin. The various technologies available for the hydrolytic or thermochemical processing of biomass, along with their products and possible pre-treatment steps, are illustrated in the accompanying diagram:Hydrolysis Biorefining Technologies
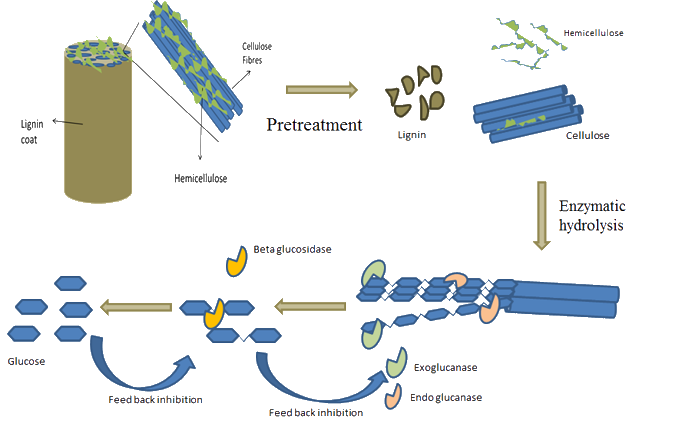
A cellulase is actually a mixture of many different enzymes each of which has a specific role in the hydrolysis of cellulose. For instance, endo-1,4-b-D-glucanases act internally within a cellulose chain at amorphous cellulose regions to cleave glyocisidc bonds, reducing the degree of polymerisation, while cellobiohydrolases degrade the cellulose chain from either the reducing or non-reducing ends and can cleave glycosidic bonds within crystalline cellulose regions, releasing cellobiose. Cellulases can be obtained from a variety of micro-organisms, including bacteria and fungi. However, aerobic fungi typically give higher growth rates and are the focus of most research.
There are numerous technologies proposed for the enzymatic hydrolysis of biomass. The most basic of these, Separate Hydrolysis and Fermentation (SHF), involves the enzymatic hydrolysis of cellulose followed by fermentation of the liberated hexoses. Pentose fermentation can occur after the distillation of the resulting beer, or with a separate stream taken from the hydrolysate of the pre-treatment process.
SSF and SSCF still require the yeast to be produced in a separate reactor, however Consolidated BioProcessing (CBP) involves all activities taking place in the same reactor, ideally with one micro-organism. CBP is considered to have the potential to offer the lowest costs for biofuels and chemicals via the enzymatic pathway.
Click here to read more about enzymatic hydrolysis, the related tests and analyses that we can undertake, and the custom processes that we can develop and optimise using enzymes.
Relevant Celignis Analysis Packages
Total Sugars in Enzyme Hydrolysate, Glucose in Enzyme Hydrolysate, Xylose in Enzyme Hydrolysate, Arabinose in Enzyme Hydrolysate, Mannose in Enzyme Hydrolysate, Galactose in Enzyme Hydrolysate, Rhamnose in Enzyme Hydrolysate, Cellobiose in Enzyme Hydrolysate, Enzymatic Hydrolysis Kinetics, Cellulose Conversion Yield, Xylan Conversion Yield, Combined Sugar Yield, Cellulose Conversion Rate, Xylan Conversion Rate
Total Sugars in Enzyme Hydrolysate, Glucose in Enzyme Hydrolysate, Xylose in Enzyme Hydrolysate, Arabinose in Enzyme Hydrolysate, Mannose in Enzyme Hydrolysate, Galactose in Enzyme Hydrolysate, Rhamnose in Enzyme Hydrolysate, Cellobiose in Enzyme Hydrolysate, Enzymatic Hydrolysis Kinetics, Cellulose Conversion Yield, Xylan Conversion Yield, Combined Sugar Yield, Cellulose Conversion Rate, Xylan Conversion Rate, Increase in Cellulose Accessibility after Pre-Treatment, Percent Increase in Cellulose Conversion Efficiency, Percent Increase in Cellulose Conversion Rate
Total Sugars in Enzyme Hydrolysate, Glucose in Enzyme Hydrolysate, Xylose in Enzyme Hydrolysate, Arabinose in Enzyme Hydrolysate, Mannose in Enzyme Hydrolysate, Galactose in Enzyme Hydrolysate, Rhamnose in Enzyme Hydrolysate, Cellobiose in Enzyme Hydrolysate, Enzymatic Hydrolysis Kinetics, Cellulose Conversion Yield, Cellulose Conversion Rate
Total Sugars in Enzyme Hydrolysate, Glucose in Enzyme Hydrolysate, Xylose in Enzyme Hydrolysate, Arabinose in Enzyme Hydrolysate, Mannose in Enzyme Hydrolysate, Galactose in Enzyme Hydrolysate, Rhamnose in Enzyme Hydrolysate, Cellobiose in Enzyme Hydrolysate, Enzymatic Hydrolysis Kinetics, Xylan Conversion Yield, Xylan Conversion Rate
(1) a bio-oil that is produced from the condensable volatile components.
(2) non-condensable gases and;
(3) biochar, a residual solid component that does not decompose under the conditions employed.
Over time, refinements have allowed process conditions and the feedstock to be manipulated in order to achieve high yields of bio-oil, gases, or biochar,
depending on the particular end product desired.
Bio-Oil
Due to the presence of water and abundance of oxygen, the heating value of the bio-oil is low compared to fossil oils and is only suitable as a heating fuel. It can, however, be used as a starting point towards a transport fuel if catalytic upgrading mechanisms are employed. Alternatively, there may be valuable chemicals that can be extracted from the bio-oil.
Celignis offers a number of analysis packages for bio-oils, these are listed below:
Biochar
At Celignis we have an array of analytical techniques and equipment suitable for the characterisation of biochars. The data we provide will assist you in determining the value of your biochar and the best use for it. Feel free to contact us to learn more.
Many potential products (such as Fischer-Tropsch diesels, alcohols, olefins, acetic acid etc.) can be synthesized from these gases with the synthesis depending on the ratio of carbon monoxide to hydrogen in the syngas. The amount of hydrogen (and hence carbon dioxide) produced can be increased via the water gas shift reaction which involves mixing steam with the syngas, since insufficient water vapour is usually released from the biomass.
Relevant Celignis Analysis Packages for Gasification
Volatile Matter, Fixed Carbon, Moisture, Ash, Carbon, Hydrogen, Nitrogen, Sulphur, Oxygen, Gross Calorific Value, Net Calorific Value, Chlorine, Ash Shrinkage Starting Temperature (Reducing), Ash Deformation Temperature (Reducing), Ash Hemisphere Temperature (Reducing), Ash Flow Temperature (Reducing), Aluminium, Calcium, Iron, Magnesium, Phosphorus, Potassium, Silicon, Sodium, Titanium
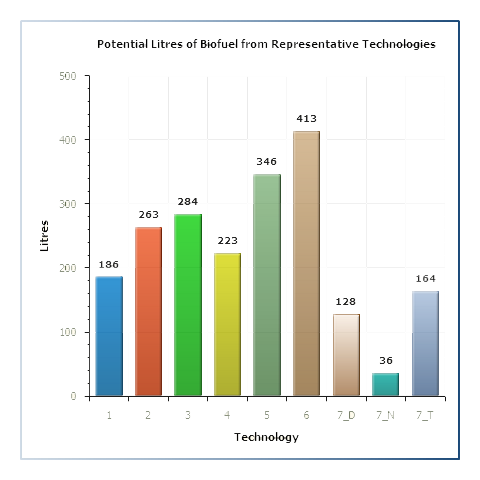
Representative Biorefining Technologies
Samples for which data regarding the lignocellulosic sugar composition have been obtained (e.g Celignis Analysis Packages: P7 (Lignocellulosic Sugars), P9 (Lignocellulosic Constituents), P10 (Lignocellulosic Constituents and Extractives), and P11 (NIR Prediction Package) will have data for potential biofuel yields from 5 different representative hydrolysis technologies.
Samples for which data regarding the heating values have been obtained (e.g. Celignis Analysis Package P40 - Combustion Package) will have data for potential biofuel yields from 2 different representative gasification technologies.
Click here for more information on these representative technologies and for details on how the biofuel yields are calculated.
weet sorghum bagasse displays many characteristics rendering it a promising substrate for lignocellulosic ethanol production. In this study, the steam pretreatment catalyst, enzymatic hydrolysis and the substrate loading for the fermentation were investigated in order to maximise the production of ethanol from the feedstock. The results deemed water as a sufficient pretreatment catalyst since the SO2 impregnation of the biomass did not produce any significant beneficial effects on the yield of ethanol produced. The preferred pretreatment and enzymatic hydrolysis conditions were incorporated in a fed-batch simultaneous saccharification and fermentation (SSF) process using pressed-only (not washed) WIS at a final solid loading of 13% (w/w) that resulted in the targeted ethanol concentration of 39 g/L with a corresponding yield of 82% of the theoretical maximum. Yeast inhibition coupled with significant glucose accumulation was observed at higher solid loadings of 16% and 20%. Ultimately, the sweet sorghum bagasse could be integrated into existing ethanol production regimes to improve the global bioenergy production. | |
Six conceptual process scenarios for the production of biobutanol from lignocellulosic biomass through acetone?butanol?ethanol (ABE) fermentation, using reported data on process performances, were developed with ASPEN Plus® V8.2 software. The six scenarios covered three fermentation strategies, i.e. batch separate hydrolysis and fermentation (SHF), continuous SHF, and batch simultaneous saccharification and fermentation (SSF) integrated with gas stripping (GS). The two downstream processing options considered were double?effect distillation (DD) and liquid?liquid extraction and distillation (LLE&D). It was found that the SSF?GS/DD scenario was the most energy efficient with a liquid fuel efficiency of 24% and an overall efficiency of 31%. This was also the scenario with the best economic outcome, with an internal rate of return (IRR) of 15% and net present value (NPV) of US$387 million. The SSF?GS/DD scenario was compared to a similar molasses process, based on the product flow rates, and it was found that the molasses process was more energy efficient with a gross energy value (GEV) of 23?MJ?kg1 butanol compared to ?117?MJ?kg1 butanol for the lignocellulosic process. In addition, the molasses?based process was more profitable with an IRR of 36% compared to 21%. However, the energy requirements for the molasses process were supplied from fossil fuels, whereas for the lignocellulose processes a portion of the feedstock was diverted to provide process energy. Improved environmental performance is therefore associated with the lignocellulosic process. | |
Biobutanol has gained attention as an alternative renewable transportation fuel for its superior fuel properties and widespread applications in chemical industry, primarily as a solvent. Conventional butanol fermentation has drawbacks that include strain degeneration, end-product toxicity, by-product formation, low butanol concentrations and high substrate cost. The complexity of Clostridium physiology and close control between sporulation phase and ABE fermentation has made it demanding to develop industrially potent strains. In addition to the isolation and engineering of superior butanol producing bacteria, the development of advanced cost-effective technologies for butanol production from feedstock like lignocellulosic biomass has become the primary research focus. High process costs associated with complex feedstocks, product toxicity and low product concentrations are few of the several bioprocess challenges involved in biobutanol production. The article aims to assess the challenges in lignocellulosic biomass to biobutanol conversion and identify key process improvements that can make biobutanol commercially viable. | |
The paper and pulp industry is one of the major industries that generate large amount of solid waste with high moisture content. Numerous opportunities exist for valorisation of waste paper sludge, although this review focuses on primary sludge with high cellulose content. The most mature options for paper sludge valorisation are fermentation, anaerobic digestion and pyrolysis. In this review, biochemical and thermal processes are considered individually and also as integrated biorefinery. The objective of integrated biorefinery is to reduce or avoid paper sludge disposal by landfilling, water reclamation and value addition. Assessment of selected processes for biorefinery varies from a detailed analysis of a single process to high level optimisation and integration of the processes, which allow the initial assessment and comparison of technologies. This data can be used to provide key stakeholders with a roadmap of technologies that can generate economic benefits, and reduce carbon wastage and pollution load. | ||
Paper sludge (PS) from the paper and pulp industry consists primarily of cellulose and ash and has significant potential for ethanol production. Thirty-seven PS samples from 11 South African paper and pulp mills exhibited large variation in chemical composition and resulting ethanol production. Simultaneous saccharification and fermentation (SSF) of PS in fed-batch culture was investigated at high solid loadings and low enzyme dosages. Water holding capacity and viscosity of the PS influenced ethanol production at elevated solid loadings of PS. High viscosity of PS from virgin pulp mills restricted the solid loading to 18% (w/w) at an enzyme dosage of 20 FPU/gram dry PS (gdPS), whereas an optimal solid loading of 27% (w/w) was achieved with corrugated recycle mill PS at 11 FPU/gdPS. Ethanol concentration and yield of virgin pulp and corrugated recycle PS were 34.2 g/L at 66.9% and 45.5 g/L at 78.2%, respectively. | |
Paper sludge samples collected from recycling mills exhibited high ash content in the range of 54.59%–65.50% and glucose concentrations between 21.97% and 31.11%. Washing the sludge reduced the total ash content to between 10.7% and 19.31% and increased the concentration of glucose, xylose and lignin. Samples were screened for ethanol production and fed-batch simultaneous saccharification and fermentation (SSF) was optimised for the washed samples that resulted in highest and lowest ethanol concentrations. Maximum ethanol concentrations of 57.31 g/L and 47.72 g/L (94.07% and 85.34% of the maximum theoretical yield, respectively) was predicted for high and low fermentative potential samples, respectively, and was experimentally achieved with 1% deviation. A generic set of process conditions were established for the conversion of high ash-containing paper sludge to ethanol. Techno-economic analysis based on three different revenue scenarios, together with Monte Carlo analysis revealed 95% probability of achieving IRR values in excess of 25% at a paper sludge feed rate of 15 t/d. Feed rates of 30 t/d and 50 t/d exhibited a cumulative probability of 100%. This study presents the technical feasibility and economic viability of paper mills expansion towards bioethanol production from paper sludge. | |
Next-generation biofuels from renewable sources have gained interest among research investigators, industrialists, and governments due to major concerns on the volatility of oil prices, climate change, and depletion of oil reserves. Biobutanol has drawn signicant attention as an alternative transportation fuel due to its superior fuel properties over ethanol. e advantages of butanol are its high energy content, better blending with gasoline, less hydroscopic nature, lower volatility, direct use in convention engines, low corrosiveness, etc. Butanol production through (acetone, butanol, and ethanol) ABE fermentation is a well-established process, but it has several drawbacks like feedstock cost, strain degeneration, product toxicity, and low product concentrations. Lignocellulosic biomass is considered as the most abundant, renewable, low-cost feedstock for biofuels. Production of butanol from lignocellulosic biomass is more complicated due to the recalcitrance of feedstock and inhibitors generated during the pretreatment and hydrolysis process. Advanced fermentation and product recovery techniques are being researched to make biobutanol industrially viable. | |
Clostridium sporogenes BE01, a non-acetone forming butanol producer, can produce hydrogen and volatile fatty acids (VFAs) during butanol fermentation from rice straw hydrolysate. Bio-electrochemical analysis revealed the changes that occurred in the redox microenvironment and electron transport mediators during fermentation at different pH and CaCO3 concentrations. CaCO3 played a very important role in enhancing the production of hydrogen, volatile fatty acids and solvents by stimulating the changes in the electron transport system. The electron transport system mediated by NAD/NADH, flavins, Fe–S clusters, protein bound FAD, and cytochrome complex in C. sporogenes BE01 was analysed by cyclic voltammetry (CV). Electrokinetic analysis revealed that the favorability for redox reactions increased with an increase in pH, and the polarization resistance reduced significantly with CaCO3 supplementation. | |
Bioethanol and biobutanol hold great promise as alternative biofuels, especially for transport sector, because they can be produced from lignocellulosic agro-industrial residues. From techno-economic point of view, the bioprocess for biofuels production should involve minimal processing steps. Consolidated bioprocessing (CBP), which combines various processing steps such as pretreatment, hydrolysis and fermentation in a single bioreactor, could be of great relevance for the production of bioethanol and biobutanol or solvents (acetone, butanol, ethanol), employing clostridia. For CBP, Clostridium holds best promise because it possesses multi-enzyme system involving cellulosome and xylanosome, which comprise several enzymes such as cellulases and xylanases. The aim of this article was to review the recent developments on enzyme systems of clostridia, especially xylanase and cellulase with an effort to analyse the information available on molecular approaches for the improvement of strains with ultimate aim to improve the efficiencies of hydrolysis and fermentation. | |
Growth inhibition kinetics of a novel non-acetone forming butanol producer, Clostridium sporogenes BE01, was studied under varying concentrations of acetic and formic acids in rice straw hydrolysate medium. Both the organic acids were considered as inhibitors as they could inhibit the growth of the bacterium, and the inhibition constants were determined to be 1.6 and 0.76 g/L, respectively, for acetic acid and formic acid. Amberlite resins—XAD 4, XAD 7, XAD 16, and an anion exchange resin—Seralite 400 were tested for the efficient removal of these acidic inhibitors along with minimal adsorption of sugars and essential minerals present in the hydrolysate. Seralite 400 was an efficient adsorbent of acids, with minimal affinity towards minerals and sugars. Butanol production was evaluated to emphasize the effect of minerals loss and acids removal by the resins during detoxification. | |
The DIBANET process chain, as a result of its patented pre-treatment stage, has significantly increased the yields of levulinic acid, formic acid, and furfural beyond what was considered to be the state of the art. By fractionating lignocellulosic biomass into its three main polymers (cellulose, hemicellulose, lignin) it has also allowed for lignin to be recovered and sold as a higher-value product. These developments have meant that the amount of acid hydrolysis residues (AHRs) that have been produced are significantly (up to 88%) less than in the Biofine process. These AHRs are required to provide process heat for DIBANET. Direct combustion is the most efficient means for doing this. If such combustion does not occur and the AHRs are instead used in other processes, e.g. pyrolysis and gasification, then more biomass will need to be purchased to fuel the core DIBANET process. The AHRs have not been proven to be superior to virgin biomass when put through these thermochemical processes. Indeed, many of the results from DIBANET Work Package 4 indicate the opposite. Hence, given that DIBANET, and the modelling of its optimal configuration, is designed on the basis of an integrated process, centred on the core element of the acid hydrolysis of biomass, then combustion is the only viable end use for the AHRs.
| ||
Biobutanol from lignocellulosic biomass has gained much attention due to several advantages over bioethanol. Though microbial production of butanol through ABE fermentation is an established technology, the use of lignocellulosic biomass as feedstock presents several challenges. In the present study, biobutanol production from enzymatic hydrolysate of acid pretreated rice straw was evaluated using Clostridium sporogenes BE01. This strain gave a butanol yield of 3.43 g/l and a total solvent yield of 5.32 g/l in rice straw hydrolysate supplemented with calcium carbonate and yeast extract. Hydrolysate was analyzed for the level of inhibitors such as acetic acid, formic acid and furfurals which affect the growth of the organism and in turn ABE fermentation. Methods for preconditioning the hydrolysate to remove toxic end products were done so as to improve the fermentation efficiency. Conditions of ABE fermentation were fine tuned resulting in an enhanced biobutanol reaching 5.52 g/l. | |
Biobutanol from lignocellulosic biomass has gained much attention due to several advantages over bioethanol. Though microbial production of butanol through ABE fermentation is an established technology, the use of lignocellulosic biomass as feedstock presents several challenges. In the present study, biobutanol production from enzymatic hydrolysate of acid pretreated rice straw was evaluated using Clostridium sporogenes BE01. This strain gave a butanol yield of 3.43 g/l and a total solvent yield of 5.32 g/l in rice straw hydrolysate supplemented with calcium carbonate and yeast extract. Hydrolysate was analyzed for the level of inhibitors such as acetic acid, formic acid and furfurals which affect the growth of the organism and in turn ABE fermentation. Methods for preconditioning the hydrolysate to remove toxic end products were done so as to improve the fermentation efficiency. Conditions of ABE fermentation were fine tuned resulting in an enhanced biobutanol reaching 5.52 g/l. | |
Aspergillus niger NII-08121/MTCC 7956 exhibited differences in expression of ?-glucosidase (BGL) in response to carbon sources provided in the medium. Activity staining with methyl umbelliferyl ?-d-glucopyranoside (MUG) indicated that four different isoforms of BGL were expressed when A. niger was grown under submerged fermentation with either lactose or cellulose, whereas only two were expressed when wheat bran or rice straw was used as the carbon source. Among the four isoforms of BGL expressed during lactose supplementation, two were found to retain 92% and 82% activity respectively in presence of 250 mM glucose in the MUG assay. The major ?-glucosidase (BGL1) was purified to homogeneity by electro elution from a Native PAGE gel. The purified 120 kDa protein was active at 50 °C and was stable for 48 h without any loss of activity. The optimum pH and temperature were 4.8 and 70 °C respectively. | |
ABE (Acetone-Butanol-Ethanol) fermentations were next only to ethanol fermentations and used to be a major industry until 1960s. Later, biological route for butanol production lost its importance owing to competition from petrochemical route, and today there is a renewed interest in ABE fermentation due to increased concerns over petroleum depletion and the increased pollution due to burning of petroleum fuels. Though the ABE fermentation process used to be operational decades back, the same technologies are not applicable today due to the lack of cost effectiveness and the nonavailability of conventional raw materials. The most feasible feedstock for butanol seems to be lignocellulose, but the problems plaguing bioethanol are also applicable for biobutanol. However, the future for biobutanol seemsbright since the Clostridia that produce ABE are capable of utilizing a range of carbon sources for growth and solvent production and also are not inhibited by the sugar degradation products generated during biomass pretreatment are being developed. Meanwhile, in the short term, advanced fermentation technologies are being developed by the expert groups which tackle problems such as low cell density, viability, and solvent sensitivity by modulations in the methods of carbon feeding, mode of culture, and in situ removal and recovery of solvents. These efforts may be developed into commercially viable technologies. | |
Rice straw is an attractive lignocellulosic material for bioethanol production since it is one of the most abundant renewable resources. It has several characteristics, such as high cellulose and hemicelluloses content that can be readily hydrolyzed into fermentable sugars. But there occur several challenges and limitations in the process of converting rice straw to ethanol. The presence of high ash and silica content in rice straw makes it an inferior feedstock for ethanol production. One of the major challenges in developing technology for bioethanol production from rice straw is selection of an appropriate pretreatment technique. The choice of pretreatment methods plays an important role to increase the efficiency of enzymatic saccharification thereby making the whole process economically viable. The present review discusses the available technologies for bioethanol production using rice straw. | |
Biomass feedstock having less competition with food crops are desirable for bio-ethanol production and such resources may not be localized geographically. A distributed production strategy is therefore more suitable for feedstock like water hyacinth with a decentralized availability. In this study, we have demonstrated the suitability of this feedstock for production of fermentable sugars using cellulases produced on site. Testing of acid and alkali pretreatment methods indicated that alkali pretreatment was more efficient in making the sample susceptible to enzyme hydrolysis. Cellulase and ?-glucosidase loading and the effect of surfactants were studied and optimized to improve saccharification. Redesigning of enzyme blends resulted in an improvement of saccharification from 57% to 71%. A crude trial on fermentation of the enzymatic hydrolysate using the common baker’s yeast Saccharomyces cerevisiae yielded an ethanol concentration of 4.4 g/L. | |