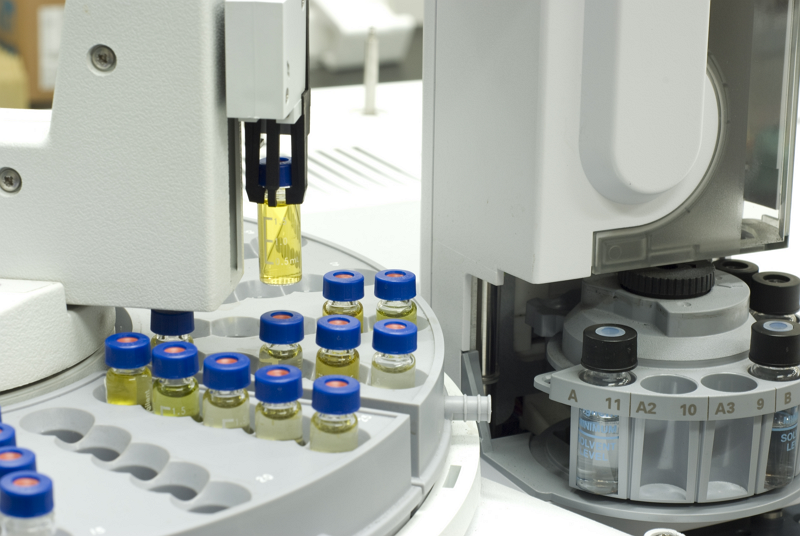
Equipment Elemental Analyser
Background
We use an Elementar MACRO cube elemental analyser. It is capable of analysing for the carbon, hydrogen, nitrogen, and sulphur contents of a sample. Up to 60 weighed samples can be placed (in folded tin foil sample boats) on a rotating carousel at the top of the system and a sequence is programmed into the software to allow for their automatic analysis.
The samples are transferred to the furnace area by means of a ball valve that rotates to receive a sample pushed towards it by the carousel. Any outside air is removed using helium and the sample is then transported to the combustion tube. This tube contains an ash crucible, with a bottom of aluminium oxide wool, that the sample drops into. It also contains differing components depending on the analysis mode that is to be run. In the CHNS mode there is a tungsten oxide (WO3) granulate that sits below the ash crucible (separated from it by corundrum balls). This is present in order to: deliver additional oxygen as a catalyst for the combustion of the sample; prevent the formation of non-volatile sulphates; and to bind alkali elements.
The combustion tube is maintained at a specified temperature, and oxygen is dosed for a set period of time in order for the following gaseous reaction products to form from the elements of interest: CO2, H2O, N2, NOx, SO2, SO3. The halogens that are present in the sample will also react to form volatile halogen compounds.
The helium carrier gas transfers the gaseous combustion products to the reduction tube which contains differing components depending on the analysis mode. In the CHNS mode, the principal filling is copper and there is also silver wool towards the top of the tube. In the reduction tube: the nitrogen oxides are completely reduced to N2 gas as a result of contact with the copper; SO3 is reduced to SO2; and any volatile halogen compounds are bound to the silver wool.
After the reduction tube the gaseous products are then passed to the separator columns zone of the system. Since N2 is not adsorbed onto the columns it is the first measuring component that enters the thermal conductivity detector (TCD). However, CO2, H2O, and SO2 are retained by specific columns for each.
Following the evolution of the nitrogen peak on the TCD the CO2 adsorption column is heated up to the CO2 desorption temperature. This results in any CO2 that was adsorbed on the column being pushed, with the carrier gas, through a water absorption column (containing SIcapent) and ultimately to the thermal conductivity detector (TCD).
The next step takes place once the carbon peak has been resolved and involves the heating of the H2O absorption column to the desorption temperature of water. The CO2 adsorption column is bypassed by this gaseous water meaning that the water reaches the TCD without passing through the Sicapent absorption column.
The SO2 adsorption column is then heated to the SO2 desorption temperature and this gaseous product bypasses both previous columns, passing through a water absorption column as it does, ultimately reaching the TCD detector. Following the analysis of this final element a cooling fan reduces the temperatures of the adsorption columns so that they are ready for the next sample. It takes approximately 10 minutes to analyse each sample.
Thermal Conductivity Detection (TCD) in Elemental Analysis
TCDs operate on the principle that the thermal conductivity properties of a gas mixture (containing the desorbed gas and the carrier gas) differs from that of the carrier gas alone.
The thermal conductivity of nitrogen is significantly less than that of helium, meaning that the thermal conductivity of a He/N2 gas mixture will be less than that of pure He.
In our Elementar MACRO cube the TCD has two sections, the analysis gas mixture flows through one (measuring cell) and the pure helium carrier gas flows through the other (reference cell). A resistance wire filament is suspended in each cell. The TCD block is heated to a constant temperature and a small current supplies additional heat to the filaments. After a period for equilibration the temperature of the filaments will attain a constant level based on: the current going through them; the conductivity of the gas passing through; and the flow rate of that gas.
Following this point, elution of analytes from the adsorption columns can take place and the carrier/analyte gas mixture will pass through the measuring cell resulting in a change in the filament temperature based on the different thermal conductivity of this mixture. The change in the resistance of the filament is measured with a bridge circuit. These filament-type detectors can operate in a constant-current mode or a constant temperature mode, with the latter mode involving the cell current varying in order to keep the filament temperature constant.
Calculation of Elemental Composition
Ultimately a peak for each of the analytes will result, and this is correlated with the absolute quantity of the element via calibrations. These calibrations relate variations in the integrated detector signal with absolute element content for samples of known weight and composition.
Quantification also requires the determination of blank values and daily factor values for each of the elements. Blank values involve running an analysis without the presence of CHNS-containing-material and measuring the determined areas for each element. This can be done over several 'sample' injections and the blank value taken as being the average of these areas for each element. The corresponding blanks are then subtracted from the elemental areas of the samples of interest.
Daily factor values are required to fine tune the instrument calibration to the room conditions at the time of analysis and to observe any drifts or trends in the performance of the device over time. These are calculated (after the determination of the blank values) by analysing sample(s) of known elemental composition. The known sample should be analysed more than once to reduce the effects that possible human/device errors may have on the weighing or analysis of that sample and, hence, incorrect determination of the daily factor. If the factor values provided by these replicates are in close agreement then their averages can be taken and used as the factor for samples that can subsequently be analysed. For long analytical sequences, and to check for drifts in device performance over the course of a day, more than one daily-factor calculating batch can be used in a sequence. In this case the samples will use the daily factor values determined from the most recently injected batch of standard samples.
It is important that the sample that is used for daily factor calculation is stable over time and has a similar elemental composition to the samples of interest. Furthermore, as is also the case for the analysis of actual samples, the areas measured for each of the elements should be within the calibration range of the calibrations available to the device or else the accuracy and reliability of analysis may be poor.
Use of the Elemental Analyser at Celignis
We use our elemental analyser to determine the carbon, hydrogen, nitrogen, and sulphur contents of biomass and liquids (e.g. bio-oils). We determine the elemental composition according to the method outlined in European Standard EN 15104:2011 ('Solid biofuels - Determination of total content of carbon, hydrogen and nitrogen - Instrumental methods').
We report the elemental composition on a dry-mass basis as well as on an as-received basis and a dry ash-free basis (providing that the ash content and as-received moisture content of the sample have also been determined). We use the calculations outlined in European Standard EN 15296:2011 ('Solid biofuels - Conversion of analytical results from one basis to another') to carry out these conversions.
We also use the elemental composition to calculate the gross calorific value and net calorific value of samples by correcting the heating value, determined using our bomb calorimeter, according to European Standard EN 14918:2009 ('Solid biofuels. Determination of calorific value'). Determination of the gross calorific value requires a correction based on the sulphur content of the sample, whilst determination of the net calorific value requires a correction of the gross calorific value based on the oxygen, nitrogen, and hydrogen contents of the sample.
We pride ourselves on the accuracy of our elemental analysis. Each sample is analysed in duplicate and we provide the compositional data for each replicate along with the average value and the standard deviation between the duplicates. Examples of the precision of our analysis can be seen here.
Analysis Packages that Use Our Elemental Analyser
Volatile Matter, Fixed Carbon, Moisture, Ash, Carbon, Hydrogen, Nitrogen, Sulphur, Oxygen, Gross Calorific Value, Net Calorific Value, Chlorine, Ash Shrinkage Starting Temperature (Reducing), Ash Deformation Temperature (Reducing), Ash Hemisphere Temperature (Reducing), Ash Flow Temperature (Reducing), Aluminium, Calcium, Iron, Magnesium, Phosphorus, Potassium, Silicon, Sodium, Titanium
Moisture, Ash Content (815C), Carbon, Hydrogen, Nitrogen, Sulphur, Oxygen, Chlorine, Volatile Matter, Fixed Carbon, Aluminium, Calcium, Iron, Magnesium, Phosphorus, Potassium, Silicon, Sodium, Titanium, Gross Calorific Value, Net Calorific Value, Ash Shrinkage Starting Temperature (Reducing), Ash Deformation Temperature (Reducing), Ash Hemisphere Temperature (Reducing), Ash Flow Temperature (Reducing)
Thernogram - Under Nitrogen, Thermogram - Under Air, Moisture, Inherent Moisture, Ash Content (815C), Carbon, Hydrogen, Nitrogen, Sulphur, Oxygen, Organic Carbon, Inorganic Carbon, Chlorine, Volatile Matter, Fixed Carbon, Aluminium, Calcium, Iron, Magnesium, Phosphorus, Potassium, Silicon, Sodium, Titanium, Gross Calorific Value, Net Calorific Value, Ash Shrinkage Starting Temperature (Reducing), Ash Deformation Temperature (Reducing), Ash Hemisphere Temperature (Reducing), Ash Flow Temperature (Reducing)
Thernogram - Under Nitrogen, Thermogram - Under Air, Moisture, Inherent Moisture, Ash Content (815C), Carbon, Hydrogen, Nitrogen, Sulphur, Oxygen, Organic Carbon, Inorganic Carbon, Chlorine, Volatile Matter, Fixed Carbon, Specific Surface Area (Nitrogen Gas Adsorption), Calcium, Iron, Magnesium, Phosphorus, Potassium, Silicon, Sodium, Titanium, Gross Calorific Value, Net Calorific Value, Ash Shrinkage Starting Temperature (Reducing), Ash Deformation Temperature (Reducing), Ash Hemisphere Temperature (Reducing), Ash Flow Temperature (Reducing)